Endophytic and epiphytic phyllosphere fungi of ... - Mycologia
Endophytic and epiphytic phyllosphere fungi of ... - Mycologia
Endophytic and epiphytic phyllosphere fungi of ... - Mycologia
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Mycologia</strong>, 100(3), 2008, pp. 387–391. DOI: 10.3852/07-110R1<br />
# 2008 by The Mycological Society <strong>of</strong> America, Lawrence, KS 66044-8897<br />
<strong>Endophytic</strong> <strong>and</strong> <strong>epiphytic</strong> <strong>phyllosphere</strong> <strong>fungi</strong> <strong>of</strong> Camellia japonica: seasonal <strong>and</strong><br />
leaf age-dependent variations<br />
Takashi Osono1 Laboratory <strong>of</strong> Forest Ecology, Graduate School <strong>of</strong><br />
Agriculture, Kyoto University, Kyoto 606-8502, Japan<br />
Abstract: Seasonal <strong>and</strong> leaf age-dependent variations<br />
in the endophytic <strong>and</strong> <strong>epiphytic</strong> <strong>phyllosphere</strong> fungal<br />
assemblages <strong>of</strong> Camellia japonica were examined <strong>and</strong><br />
compared. Live leaves <strong>of</strong> C. japonica were collected in<br />
four seasons (May, Aug, Nov, Feb), <strong>and</strong> <strong>fungi</strong> were<br />
isolated from healthy-looking leaves <strong>of</strong> 0, 1, 2 <strong>and</strong> 3 y<br />
old. The infection rate <strong>and</strong> total number <strong>of</strong> endophytic<br />
<strong>fungi</strong> increased May–Feb, <strong>and</strong> species richness<br />
<strong>of</strong> endophytes increased as leaves aged. In contrast<br />
the infection rate <strong>of</strong> <strong>epiphytic</strong> <strong>fungi</strong> was 100% for all<br />
leaf ages at every sampling date. The total number <strong>of</strong><br />
<strong>epiphytic</strong> <strong>fungi</strong> isolated was greatest in May <strong>and</strong><br />
lowest in Aug. The species richness <strong>of</strong> epiphytes did<br />
not differ significantly by season or leaf age. Eight<br />
fungal species were recorded as major <strong>phyllosphere</strong><br />
<strong>fungi</strong> <strong>of</strong> C. japonica. Seasonal variations were detected<br />
for the frequencies <strong>of</strong> Colletotrichum gloeosporioides, C.<br />
acutatum, <strong>and</strong> epiphytes Pestalotiopsis sp.1, Aureobasidium<br />
pullulans, Phoma sp.1 <strong>and</strong> Ramichloridium sp.,<br />
whereas the frequency <strong>of</strong> the endophyte Geniculosporium<br />
sp.1 varied with leaf age. The frequency <strong>of</strong> the<br />
epiphyte Cladosporium cladosporioides varied with<br />
both season <strong>and</strong> leaf age.<br />
Key words: endophytes, epiphytes, fungal<br />
assemblage, leaves, season<br />
INTRODUCTION<br />
The <strong>phyllosphere</strong> is the living leaf as a whole,<br />
including the interior <strong>and</strong> surface (Carroll et al<br />
1977), which provides habitats for a variety <strong>of</strong><br />
microorganisms. Phyllosphere <strong>fungi</strong> include endophytes<br />
<strong>and</strong> epiphytes that colonize the interior or<br />
surface <strong>of</strong> leaves, respectively (Petrini 1991). Seasonal<br />
<strong>and</strong> leaf age-dependent variations in the endophytic<br />
<strong>and</strong> <strong>epiphytic</strong> <strong>phyllosphere</strong> <strong>fungi</strong> are an important<br />
aspect <strong>of</strong> <strong>phyllosphere</strong> ecology <strong>and</strong> have been<br />
investigated on the leaves <strong>of</strong> evergreen (Ruscoe<br />
1971, Cabral 1985, Hata et al 1998) <strong>and</strong> deciduous<br />
trees (Breeze <strong>and</strong> Dix 1981, Wilson <strong>and</strong> Carroll 1994,<br />
Sahashi et al 1999, 2000, Kaneko et al 2003). Some<br />
studies have compared both endophytic <strong>and</strong> epiphyt-<br />
Accepted for publication 20 March 2008.<br />
1 Corresponding author. E-mail: fujijun@kais.kyoto-u.ac.jp<br />
387<br />
ic fungal assemblages on single leaves at the same<br />
time (Wildman <strong>and</strong> Parkinson 1978, Legault et al<br />
1989a, b, Osono 2002, 2007, Osono <strong>and</strong> Mori 2003,<br />
2004, Santamariá <strong>and</strong> Bayman 2005), but only limited<br />
information is available regarding the differences in<br />
the seasonal <strong>and</strong> leaf age-dependent dynamics <strong>of</strong><br />
endophytes <strong>and</strong> epiphytes (Ruscoe 1971, Cabral 1985,<br />
Osono <strong>and</strong> Mori 2005). Ruscoe (1971) reported that<br />
both endophytes <strong>and</strong> epiphytes rarely varied with<br />
season or leaf age. Conversely Cabral (1985) <strong>and</strong><br />
Osono <strong>and</strong> Mori (2005) found that both endophytes<br />
<strong>and</strong> epiphytes varied with season <strong>and</strong>/or leaf age.<br />
The purpose <strong>of</strong> this study was to examine <strong>and</strong><br />
compare the seasonal <strong>and</strong> leaf age-dependent variations<br />
in the endophytic <strong>and</strong> <strong>epiphytic</strong> <strong>phyllosphere</strong><br />
fungal assemblages <strong>of</strong> Camellia japonica L. I investigated<br />
(i) the infection rates, number <strong>of</strong> <strong>fungi</strong><br />
isolated, species richness <strong>of</strong> <strong>phyllosphere</strong> <strong>fungi</strong> <strong>and</strong><br />
(ii) the relative importance <strong>of</strong> season <strong>and</strong> leaf age to<br />
the occurrence <strong>of</strong> major fungal species in the <strong>phyllosphere</strong>.<br />
Camellia japonica is an evergreen broadleaf<br />
tree <strong>and</strong> is one <strong>of</strong> the dominant trees in secondary<br />
forests in western Japan.<br />
MATERIALS AND METHODS<br />
Study site.—The site was in the Oharano Forest Park <strong>of</strong><br />
Kyoto City (34u579N, 135u379E, 400 m a.s.l.), Japan. The<br />
mean annual temperature is 15.3 C <strong>and</strong> the annual<br />
precipitation is 1581.1 mm at the Kyoto Weather Station,<br />
about 12 km from the study site. The site was in a<br />
mountainous area <strong>of</strong> temperate forest, which was harvested<br />
repeatedly for fuel production until the fuel revolution in<br />
the 1950s. The site has been intact <strong>and</strong> undisturbed since<br />
then (Ishikawa et al 2007). The plot, 20 3 10 m, was laid out<br />
within a temperate secondary forest in which Quercus<br />
serrata Murr., C. japonica <strong>and</strong> Pinus densiflora Sieb. et Zucc.<br />
dominated in numbers <strong>and</strong> basal area. Most C. japonica do<br />
not reach the forest canopy <strong>and</strong> constitute the subcanopy.<br />
Sample collection.—Healthy-looking leaves <strong>of</strong> C. japonica<br />
were collected from st<strong>and</strong>ing trees within the plot in May,<br />
Aug <strong>and</strong> Nov 2004 <strong>and</strong> Feb 2005. On each sampling<br />
occasion five branches that included leaves <strong>of</strong> the four age<br />
classes (ages 0, 1, 2 <strong>and</strong> 3 y) were cut from five r<strong>and</strong>omly<br />
chosen trees, one branch per tree, at an approximate height<br />
<strong>of</strong> 6 m. The ages <strong>of</strong> the leaves were determined by<br />
examining the annual bud scars <strong>and</strong> counting the age from<br />
the apex <strong>of</strong> the twigs. Two leaves were selected arbitrarily<br />
from each age class within each branch, <strong>and</strong> a total <strong>of</strong> 40<br />
leaves (five trees 3 one branch 3 four age classes 3 two<br />
leaves) were collected on each sampling occasion. The
388 MYCOLOGIA<br />
leaves were placed in paper bags <strong>and</strong> taken to the<br />
laboratory.<br />
Four leaf disks were punched from each sampled leaf, two<br />
disks from the edge <strong>and</strong> another two from the central part<br />
<strong>of</strong> the leaves, avoiding the primary vein, with a sterile cork<br />
borer (5.5 mm diam). Two disks, one from the edge <strong>and</strong><br />
one from the central part <strong>of</strong> the leaf, were used to isolate<br />
endophytic <strong>fungi</strong> after surface disinfection, <strong>and</strong> another two<br />
disks were used to isolate <strong>epiphytic</strong> <strong>fungi</strong> after washing the<br />
disks, as described below. Fungi were isolated from a total <strong>of</strong><br />
160 leaf disks on each sampling occasion within 24 h <strong>of</strong><br />
collection.<br />
Fungal isolation <strong>and</strong> identification.—A surface disinfection<br />
method (Kinkel <strong>and</strong> Andrews 1988, Hata et al 1998) <strong>and</strong> a<br />
modified washing method (Harley <strong>and</strong> Waid 1955, Tokumasu<br />
1996) were used according to Osono (2005). For<br />
surface disinfection the leaf disks were submerged in 70%<br />
ethanol (v/v) for 1 min to wet the surface, then surface<br />
disinfected for 15 s in a solution <strong>of</strong> 15% hydrogen peroxide<br />
(v/v) <strong>and</strong> then submerged again for 1 min in 70% ethanol.<br />
The disks were rinsed with sterile, distilled water, transferred<br />
to sterile filter paper in Petri dishes (9 cm diam) <strong>and</strong><br />
dried 24 h to suppress vigorous bacterial growth after<br />
plating (Widden <strong>and</strong> Parkinson 1973). The disks were<br />
placed on 9 cm Petri dishes containing LCA media (Miura<br />
<strong>and</strong> Kudo 1970), two disks per plate. LCA contains glucose<br />
0.1%, KH2PO4 0.1%, MgSO4.7H2O 0.02%, KCl 0.02%,<br />
NaNO 3 0.2%, yeast extract 0.02% <strong>and</strong> agar 1.3% (w/v).<br />
LCA was used because its low glucose content suppresses<br />
the overgrowth <strong>of</strong> fast-growing species <strong>and</strong> because LCA<br />
induces sporulation, which is useful for fungal identification<br />
(Osono <strong>and</strong> Takeda 1999).<br />
For modified washing disks were washed in a sterile test<br />
tube <strong>and</strong> agitated in a vertical shaker 1.5 min to isolate<br />
<strong>fungi</strong> growing on the surface. The disks were washed serially<br />
in two changes <strong>of</strong> 0.005% aerosol-OT (di-2-ethylhexyl<br />
sodium sulfosuccinate) solution (w/v) <strong>and</strong> rinsed four<br />
times with sterile distilled water. The washed disks were<br />
treated in the same manner as that used in the plating-out<br />
procedure described for the surface disinfected leaves.<br />
The plates were incubated at 20 C in the dark <strong>and</strong><br />
observed at 1, 4 <strong>and</strong> 8 wk after surface disinfection or<br />
washing (Osono <strong>and</strong> Takeda 1999). Any hyphae or spores<br />
on the plates were subcultured on fresh LCA, incubated <strong>and</strong><br />
identified to species or genus. Identification was based on<br />
micromorphological observations, with reference to<br />
Domsch et al (1980) <strong>and</strong> Ellis (1971, 1976).<br />
Statistical analysis.—The infection rate was calculated as<br />
the number <strong>of</strong> leaf disks with any fungus isolated divided by<br />
the total number <strong>of</strong> disks (10) that were surface disinfected<br />
or washed from each leaf position in each age class at each<br />
sampling. The total number <strong>of</strong> <strong>fungi</strong> isolated <strong>and</strong> the total<br />
number <strong>of</strong> species were recorded for the fungal assemblages<br />
in each 10-disk set. The frequency <strong>of</strong> a single species was<br />
calculated as the percentage <strong>of</strong> the number <strong>of</strong> disks<br />
containing the species out <strong>of</strong> the 10 disks tested, which<br />
had been either surface disinfected or washed, at each leaf<br />
position in each age class at each sampling. Fungal species<br />
were regarded as major species when they were isolated<br />
from disks by either method at a frequency greater than<br />
12.5% on any sampling occasion. Only the results for the<br />
frequent species are shown in the present study.<br />
Kruskal-Wallis test was used to evaluate differences in the<br />
infection rate, total number <strong>of</strong> <strong>fungi</strong> isolated, number <strong>of</strong><br />
species <strong>of</strong> endophytes <strong>and</strong> epiphytes <strong>and</strong> the frequencies <strong>of</strong><br />
individual species among four leaf ages (0, 1, 2 or 3 y) <strong>and</strong><br />
four seasons (May, Aug, Nov or Feb). Mann-Whitney U test<br />
was used to evaluate difference between two leaf positions<br />
(edge or center). The analyses were performed on<br />
Macintosh with Systat ver. 5.2 (Systat 1992).<br />
Sørensen’s quotient <strong>of</strong> similarity (QS) was calculated to<br />
examine the similarity <strong>of</strong> fungal assemblages in leaf<br />
interiors <strong>and</strong> on leaf surfaces <strong>and</strong> to compare the similarity<br />
with those previously reported in other host trees (Osono<br />
<strong>and</strong> Mori 2004):<br />
QS ~ 2a= ð2azbzcÞ where a is the number <strong>of</strong> common species <strong>and</strong> b <strong>and</strong> c<br />
are the numbers <strong>of</strong> species specific to the interior <strong>and</strong><br />
the surface, respectively.<br />
RESULTS<br />
Phyllosphere fungal assemblages.—A total <strong>of</strong> 79 species<br />
were isolated from C. japonica leaves—44 endophytic<br />
species, 52 <strong>epiphytic</strong> species with 17 species common<br />
to both habitats. Sørensen’s QS for the endophytic<br />
<strong>and</strong> <strong>epiphytic</strong> fungal assemblages was 0.35.<br />
Infection rate <strong>and</strong> total number <strong>of</strong> endophytic<br />
<strong>fungi</strong> varied significantly with season, generally<br />
increasing May—Feb (TABLE I). Species richness <strong>of</strong><br />
endophytes increased significantly as leaves aged<br />
(TABLE I). In contrast the infection rates <strong>of</strong> <strong>epiphytic</strong><br />
<strong>fungi</strong> were 100% for all leaf ages at every sampling<br />
date (TABLE I). Total number <strong>of</strong> <strong>epiphytic</strong> <strong>fungi</strong><br />
isolated varied significantly with season <strong>and</strong> was<br />
greatest in May <strong>and</strong> lowest in Aug (TABLE I). Species<br />
richness <strong>of</strong> epiphytes did not differ significantly by<br />
season or leaf age (TABLE I). No significant differences<br />
were found for the infection rate, the total number<br />
<strong>of</strong> <strong>fungi</strong> isolated <strong>and</strong> the number <strong>of</strong> species <strong>of</strong><br />
endophytic <strong>and</strong> <strong>epiphytic</strong> <strong>fungi</strong> between leaf edge<br />
<strong>and</strong> leaf center (TABLE I).<br />
Variation in major species.—Eight fungal species were<br />
regarded as major <strong>phyllosphere</strong> <strong>fungi</strong> (TABLE II). The<br />
frequencies <strong>of</strong> Colletotrichum gloeosporioides (both<br />
endophytic <strong>and</strong> <strong>epiphytic</strong>), Colletotrichum acutatum,<br />
Pestalotiopsis sp.1, Cladosporium cladosporioides, Aureobasidium<br />
pullulans, Phoma sp.1 <strong>and</strong> Ramichloridium<br />
sp. varied significantly with season (TABLE II). Colletotrichum<br />
gloeosporioides, Pestalotiopsis sp.1, Clad. cladosporioides<br />
<strong>and</strong> Phoma sp.1 were frequent in May; A.<br />
pullulans <strong>and</strong> Ramichloridium sp. were frequent in<br />
Aug; C. gloeosporioides, C. acutatum, Pestalotiopsis sp.1,
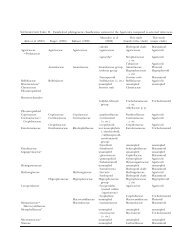
TABLE I. Effects <strong>of</strong> season, leaf age, <strong>and</strong> leaf position on the infection rate, total number <strong>of</strong> <strong>fungi</strong> isolated, <strong>and</strong> species<br />
richness <strong>of</strong> endophytic <strong>and</strong> <strong>epiphytic</strong> <strong>phyllosphere</strong> <strong>fungi</strong> in Camellia japonica. Data represent the mean values <strong>of</strong> leaf edge <strong>and</strong><br />
leaf center subsamples collected from 10 disks each<br />
Season Leaf age<br />
Clad. cladosporioides <strong>and</strong> A. pullulans were frequent<br />
in Nov; <strong>and</strong> C. gloeosporioides, Pestalotiopsis sp.1, <strong>and</strong><br />
Clad. cladosporioides were frequent in Feb (TABLE II).<br />
The frequencies <strong>of</strong> Geniculosporium sp.1 <strong>and</strong> Clad.<br />
cladosporioides varied significantly with leaf age <strong>and</strong><br />
generally increased as leaves aged (TABLE II). No<br />
significant differences were found for the frequencies<br />
<strong>of</strong> major species between leaf edge <strong>and</strong> leaf center<br />
(TABLE II).<br />
DISCUSSION<br />
Infection<br />
rate (%)<br />
This study investigated <strong>and</strong> compared the endophytic<br />
<strong>and</strong> <strong>epiphytic</strong> <strong>phyllosphere</strong> <strong>fungi</strong> <strong>of</strong> C. japonica. The<br />
finding that the total species number was greater on<br />
the leaf surface (52) than in the interior (44) is<br />
consistent with the results <strong>of</strong> previous studies (summarized<br />
in Osono <strong>and</strong> Mori 2004). Sørensen’s QS <strong>of</strong><br />
0.35 for the endophytic <strong>and</strong> <strong>epiphytic</strong> fungal assemblages<br />
is intermediate in the range (0.12–0.79) <strong>of</strong><br />
previous studies on forest tree leaves (summarized in<br />
Osono <strong>and</strong> Mori 2004).<br />
Koide et al (2005) found C. gloeosporioides <strong>and</strong><br />
Geniculosporium sp. 1 to be frequent endophytes <strong>of</strong> C.<br />
japonica leaves at the same study site, supporting our<br />
findings. Koide et al (2005) also isolated rhytismataceous<br />
<strong>fungi</strong> as endophytes <strong>of</strong> C. japonica leaves, but in their<br />
Endophytes Epiphytes<br />
Total number<br />
<strong>of</strong> <strong>fungi</strong><br />
Number<br />
<strong>of</strong> species<br />
Infection<br />
rate (%)<br />
Total number<br />
<strong>of</strong> <strong>fungi</strong><br />
Number<br />
<strong>of</strong> species<br />
May 0 55 5.5 2.5 100 16.5 7.5<br />
1 65 6.0 4.0 100 17.0 7.5<br />
2 80 8.0 6.5 100 21.5 8.0<br />
3 90 10.5 6.5 100 22.5 8.5<br />
Aug 0 70 7.0 3.5 100 11.5 5.0<br />
1 60 6.0 4.0 100 14.5 6.5<br />
2 70 6.5 6.0 100 13.5 9.5<br />
3 75 7.5 5.5 100 14.5 7.0<br />
Nov 0 100 9.5 4.5 100 15.0 7.5<br />
1 95 12.0 6.5 100 15.0 7.0<br />
2 100 10.5 6.5 100 17.0 5.0<br />
3 100 12.0 6.5 100 17.5 7.5<br />
Feb 0 80 8.0 6.0 100 14.0 7.0<br />
1 100 11.5 6.0 100 15.0 6.5<br />
2 95 13.5 8.0 100 16.0 8.5<br />
3 100 10.0 7.0 100 17.0 7.5<br />
Probability Season ,0.001 0.002 0.09 na1 0.004 0.55<br />
Age 0.37 0.29 0.01 na 0.11 0.37<br />
Leaf position 0.34 0.73 0.59 na 0.83 0.86<br />
1 Not applied.<br />
OSONO: FUNGI OF CAMELLIA JAPONICA 389<br />
study the frequency <strong>of</strong> occurrence <strong>of</strong> these <strong>fungi</strong> was<br />
lower in live leaves than in newly shed leaves. The lack <strong>of</strong><br />
rhytismataceous <strong>fungi</strong> in the present study can be partly<br />
due to the method <strong>of</strong> fungal isolation that might have<br />
favored fast growing fungal species <strong>and</strong> to the relatively<br />
large leaf disks we used to isolate <strong>fungi</strong> (5.5 mm diam).<br />
Some species also might have been excluded in the<br />
present study due to the 24 h delay in processing <strong>and</strong><br />
24 h period <strong>of</strong> drying disks before plating them.<br />
Patterns <strong>of</strong> change in the infection rate, total<br />
number <strong>of</strong> <strong>fungi</strong> isolated <strong>and</strong> species richness<br />
differed between endophytes <strong>and</strong> epiphytes (TA-<br />
BLE I), indicating that different patterns <strong>of</strong> fungal<br />
succession occur in the two leaf habitats. Species<br />
richness <strong>of</strong> fungal endophytes was low at leaf<br />
emergence (i.e. on leaves <strong>of</strong> age 0 in May) <strong>and</strong><br />
increased as the leaves aged. A similar pattern <strong>of</strong><br />
colonization <strong>of</strong> the leaf interior by endophytic <strong>fungi</strong><br />
has been reported for Pseudotsuga menziesii (Mirb.)<br />
Franco (Stone 1987) <strong>and</strong> Pinus spp. (Hata et al 1998).<br />
In contrast colonization by <strong>epiphytic</strong> <strong>fungi</strong> began<br />
shortly after leaf emergence because the infection<br />
rate <strong>and</strong> species richness were already at or near their<br />
maximum values in the youngest leaves sampled (age<br />
0 in May). This colonization <strong>of</strong> leaf surfaces by<br />
<strong>epiphytic</strong> <strong>fungi</strong> is consistent with the result <strong>of</strong> Osono<br />
<strong>and</strong> Mori (2005).
390 MYCOLOGIA<br />
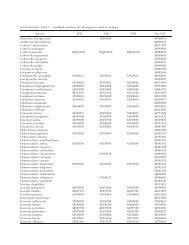
TABLE II. Effects <strong>of</strong> season, leaf age, <strong>and</strong> leaf position on the frequency <strong>of</strong> occurrence <strong>of</strong> endophytic <strong>and</strong> <strong>epiphytic</strong><br />
<strong>phyllosphere</strong> <strong>fungi</strong> in Camellia japonica. Data represent the mean values <strong>of</strong> leaf edge <strong>and</strong> leaf center subsamples collected<br />
from ten disks each<br />
Season<br />
Leaf<br />
age<br />
Colletotrichumgloeosporioides<br />
The total number <strong>of</strong> endophytic <strong>fungi</strong> isolated was<br />
lower in May <strong>and</strong> Aug than in Nov <strong>and</strong> Feb, whereas<br />
that <strong>of</strong> <strong>epiphytic</strong> <strong>fungi</strong> was lower in Aug than in May<br />
(TABLE I). This difference in seasonal pattern can be<br />
partly related to the seasonal behavior <strong>of</strong> major<br />
endophytes <strong>and</strong> epiphytes. The decrease <strong>of</strong> the<br />
endophytes C. gloeosporioides <strong>and</strong> C. acutatum in<br />
Aug (TABLE II) <strong>and</strong> the low infection by endophytes<br />
in younger leaves in May (discussed above) can<br />
account for the decrease <strong>of</strong> total number <strong>of</strong> endophytic<br />
<strong>fungi</strong> in these seasons. The decrease <strong>of</strong> C.<br />
gloeosporioides in Aug has been reported in deciduous<br />
tree leaves in Japan (Terashita 1973). Similarly only<br />
two <strong>of</strong> the common <strong>epiphytic</strong> species (A. pullulans<br />
<strong>and</strong> Ramichloridium sp.) on C. japonica leaves<br />
occurred more frequently in Aug, whereas the other<br />
four common <strong>fungi</strong> occurred more <strong>of</strong>ten in May. The<br />
higher incidence <strong>of</strong> A. pullulans <strong>and</strong> Ramichloridium<br />
sp. in Aug might be related to the dark pigmentation<br />
<strong>of</strong> hyphae in these species, thus conferring greater<br />
competitive advantage over more lightly pigmented<br />
species during hot, dry summer conditions (Butler<br />
<strong>and</strong> Day 1998).<br />
The occurrence <strong>of</strong> six <strong>of</strong> the eight major <strong>fungi</strong> <strong>of</strong> C.<br />
japonica leaves varied with season, one endophyte<br />
Endophytes Epiphytes<br />
Geniculosporium<br />
sp.1<br />
ColletotriColletotrichumacuchumgloeostatumporioides Pestalotiopsis<br />
sp.1<br />
Cladosporiumcladosporioides<br />
Aureobasidiumpullulans<br />
Phoma<br />
sp.1<br />
Ramichloridium<br />
sp.<br />
May 0 40 0 0 10 0 25 25 35 0<br />
1 25 5 5 10 15 55 0 50 0<br />
2 20 10 0 25 40 70 25 20 0<br />
3 20 15 0 25 50 60 15 30 0<br />
Aug. 0 25 0 0 5 0 5 60 0 25<br />
1 5 20 0 5 5 10 55 0 45<br />
2 10 0 0 5 5 10 40 5 20<br />
3 0 15 0 5 30 35 25 0 0<br />
Nov. 0 30 5 30 40 25 10 35 5 5<br />
1 50 5 25 10 50 15 40 0 0<br />
2 35 15 10 30 50 45 35 0 5<br />
3 55 5 0 20 80 25 10 0 0<br />
Feb. 0 20 0 5 10 45 0 10 5 0<br />
1 25 30 0 5 80 20 0 5 0<br />
2 40 5 0 5 45 30 10 10 5<br />
3 25 20 0 20 55 45 5 5 5<br />
Probability Season 0.005 0.77 0.007 0.03 0.004 0.008 ,0.001 ,0.001 0.003<br />
Age 0.92 0.04 0.20 0.44 0.06 0.006 0.31 0.95 0.48<br />
Leaf<br />
position<br />
0.41 0.35 0.70 0.054 0.66 0.9 0.88 0.95 0.93<br />
Kruskal-Wallis test was used to examine the effect <strong>of</strong> season <strong>and</strong> leaf age <strong>and</strong> Mann-Whitney test for the effect <strong>of</strong> leaf position.<br />
varied with leaf age <strong>and</strong> one epiphyte with both<br />
(TABLE II). Two studies have examined the effects <strong>of</strong><br />
season <strong>and</strong> leaf age on the occurrence <strong>of</strong> <strong>phyllosphere</strong><br />
<strong>fungi</strong> in evergreen broadleaf tree species.<br />
Ruscoe (1971) recorded seven major <strong>phyllosphere</strong><br />
<strong>fungi</strong> <strong>of</strong> Noth<strong>of</strong>agus truncata, <strong>and</strong> one epiphyte<br />
(Pestalotia funerea) showed seasonal variation, whereas<br />
the other six <strong>fungi</strong> varied with neither season nor<br />
leaf age. On Eucalyptus viminalis leaves (Cabral 1985)<br />
two epiphytes (Clad. cladosporioides <strong>and</strong> Epicoccum<br />
nigrum) <strong>and</strong> an endophyte (Zoellneria eucalypti)<br />
showed seasonal variation, two endophytes (Alternaria<br />
Alternaria alternata <strong>and</strong> Coccomyces maritinae) varied<br />
with leaf age <strong>and</strong> one endophyte (Coniothyrium sp.)<br />
varied with both season <strong>and</strong> leaf age. Comparisons<br />
among previous studies <strong>and</strong> this study suggest that<br />
seasonal variation might influence more frequently<br />
than leaf age, but consistent patterns <strong>of</strong> <strong>phyllosphere</strong><br />
fungal colonization <strong>of</strong> evergreen leaves due to<br />
seasonal <strong>and</strong> leaf age variation are difficult to predict.<br />
For example Clad. cladosporioides <strong>and</strong> A. pullulans<br />
showed different patterns <strong>of</strong> seasonal <strong>and</strong> leaf agedependent<br />
variations on different hosts. More detailed<br />
analyses <strong>of</strong> the seasonal <strong>and</strong> leaf age-dependent<br />
changes in leaf environmental conditions might
provide further insights into the dynamics <strong>of</strong> endophytic<br />
<strong>and</strong> <strong>epiphytic</strong> <strong>phyllosphere</strong> <strong>fungi</strong> on forest<br />
trees.<br />
ACKNOWLEDGMENTS<br />
We thank Dr S. Tokumasu <strong>and</strong> Dr D. Hirose for their<br />
helpful identification <strong>of</strong> <strong>fungi</strong>, Dr A. Mori for his comments<br />
on statistical analysis <strong>and</strong> Ms K. Koide for her valuable<br />
discussion. This study received partial financial support<br />
from the Japanese Ministry <strong>of</strong> Education, Culture <strong>and</strong><br />
Sports (No. 14760099).<br />
LITERATURE CITED<br />
Breeze EM, Dix NJ. 1981. Seasonal analysis <strong>of</strong> the fungal<br />
community on Acer platanoides leaves. Trans Br Mycol<br />
Soc 77:321–328.<br />
Butler MJ, Day AW. 1998. Fungal melanins: a review. Can J<br />
Microbiol 44:1115–1136.<br />
Cabral D. 1985. Phyllosphere <strong>of</strong> Eucalyptus viminalis:<br />
dynamics <strong>of</strong> fungal populations. Trans Br Mycol Soc<br />
85:501–511.<br />
Carroll GC, Muller EM, Sutton BC. 1977. Preliminary<br />
studies on the incidence <strong>of</strong> needle endophytes in some<br />
European conifers. Sydowia 29:87–103.<br />
Domsch KH, Gams W, Anderson TH. 1980. Compendium<br />
<strong>of</strong> soil <strong>fungi</strong>. Volume I. Eching: IHW-Verlag. 860 p.<br />
Ellis MB. 1971. Dematiaceous hyphomycetes. Oxon: CAB<br />
International. 608 p.<br />
———. 1976. More dematiaceous hyphomycetes. Oxon:<br />
CAB International. 507 p.<br />
Harley JL, Waid JS. 1955. A method <strong>of</strong> studying active<br />
mycelia on living roots <strong>and</strong> other surfaces in the soil.<br />
Trans Br Mycol Soc 38:104–118.<br />
Hata K, Futai K, Tsuda M. 1998. Seasonal <strong>and</strong> needle agedependent<br />
changes <strong>of</strong> the endophytic mycobiota in<br />
Pinus thunbergii <strong>and</strong> Pinus densiflora needles. Can J<br />
Bot 76:245–250.<br />
Ishikawa H, Osono T, Takeda H. 2007. Effects <strong>of</strong> clearcutting<br />
on decomposition processes in leaf litter <strong>and</strong><br />
the nitrogen <strong>and</strong> lignin dynamics in a temperate<br />
secondary forest. J For Res 12:247–254.<br />
Kaneko R, Kakishima M, Tokumasu S. 2003. The seasonal<br />
occurrence <strong>of</strong> endophytic fungus, Mycosphaerella buna,<br />
in Japanese beech, Fagus crenata. Mycoscience 44:277–<br />
281.<br />
Kinkel LL, Andrews JH. 1988. Disinfestation <strong>of</strong> living leaves<br />
by hydrogen peroxide. Trans Br Mycol Soc 91:523–528.<br />
Koide K, Osono T, Takeda H. 2005. Colonization <strong>and</strong> lignin<br />
decomposition <strong>of</strong> Camellia japonica leaf litter by<br />
endophytic <strong>fungi</strong>. Mycoscience 46:280–286.<br />
Legault D, Dessureault M, Laflamme G. 1989a. Myc<strong>of</strong>lore<br />
des aiguilles de Pinus banksiana et Pinus resinosa I.<br />
Champignons endophytes. Can J Bot 67:2052–2060.<br />
———, ———, ———. 1989b. Myc<strong>of</strong>lora <strong>of</strong> Pinus bank-<br />
OSONO: FUNGI OF CAMELLIA JAPONICA 391<br />
siana <strong>and</strong> Pinus resinosa needles II. Epiphytic <strong>fungi</strong>.<br />
Can J Bot 67:2061–2065.<br />
Miura K, Kudo M. 1970. An agar-medium for aquatic<br />
hyphomycetes. Trans Mycol Soc Japan 11:116–118.<br />
Osono T. 2002. Phyllosphere <strong>fungi</strong> on leaf litter <strong>of</strong> Fagus<br />
crenata: occurrence, colonization, <strong>and</strong> succession. Can<br />
J Bot 80:460–469.<br />
———. 2005. Colonization <strong>and</strong> succession <strong>of</strong> <strong>fungi</strong> during<br />
decomposition <strong>of</strong> Swida controversa leaf litter. <strong>Mycologia</strong><br />
97:589–597.<br />
———. 2007. <strong>Endophytic</strong> <strong>and</strong> <strong>epiphytic</strong> <strong>phyllosphere</strong> <strong>fungi</strong><br />
<strong>of</strong> red-osier dogwood (Cornus stolonifera) in British<br />
Columbia. Mycoscience 48:47–52.<br />
———, Mori A. 2003. Colonization <strong>of</strong> Japanese beech leaves<br />
by <strong>phyllosphere</strong> <strong>fungi</strong>. Mycoscience 44:437–441.<br />
———, ———. 2004. Distribution <strong>of</strong> <strong>phyllosphere</strong> <strong>fungi</strong><br />
within the canopy <strong>of</strong> giant dogwood. Mycoscience 45:<br />
161–168.<br />
———, ———. 2005. Seasonal <strong>and</strong> leaf age-dependent<br />
changes in occurrence <strong>of</strong> <strong>phyllosphere</strong> <strong>fungi</strong> in giant<br />
dogwood. Mycoscience 46:273–279.<br />
———, Takeda H. 1999. A methodological survey on<br />
incubation <strong>of</strong> <strong>fungi</strong> on leaf litter <strong>of</strong> Fagus crenata.<br />
Appl For Sci Kansai 8:103–108.<br />
Petrini O. 1991. Fungal endophytes <strong>of</strong> tree leaves. In:<br />
Andrews JH, Hirano SS., eds., eds. Microbial ecology <strong>of</strong><br />
leaves. New York: Springer Verlag. p 179–197.<br />
Ruscoe QW. 1971. Myc<strong>of</strong>lora <strong>of</strong> living <strong>and</strong> dead leaves <strong>of</strong><br />
Noth<strong>of</strong>agus truncata. Trans Br Mycol Soc 56:463–474.<br />
Sahashi N, Kubono T, Miyasawa Y, Ito S. 1999. Temporal<br />
variations in isolation frequency <strong>of</strong> endophytic <strong>fungi</strong> <strong>of</strong><br />
Japanese beech. Can J Bot 77:197–202.<br />
———, Miyasawa Y, Kubono T, Ito S. 2000. Colonization <strong>of</strong><br />
beech leaves by two endophytic <strong>fungi</strong> in northern<br />
Japan. For Pathol 30:77–86.<br />
Santamariá J, Bayman P. 2005. Fungal epiphytes <strong>and</strong><br />
endophytes <strong>of</strong> c<strong>of</strong>fee leaves (C<strong>of</strong>fea arabica). Microb<br />
Ecol 50:1–8.<br />
Stone JK. 1987. Initiation <strong>and</strong> development <strong>of</strong> latent<br />
infections by Rhabdocline parkeri on Douglas-fir. Can J<br />
Bot 57:2800–2811.<br />
Systat.. 1992. Statistics, version 5.2. Evanston: Systat Inc.<br />
Terashita T. 1973. Studies <strong>of</strong> an anthracnose fungus on<br />
broad-leaved trees in Japan, with special reference to<br />
the latency <strong>of</strong> the fungus. Bull Gov For Exp Sta 252:1–<br />
85. (in Japanese with English abstract)<br />
Tokumasu S. 1996. Myc<strong>of</strong>loral succession on Pinus densiflora<br />
needles on a moder site. Mycoscience 37:313–<br />
321.<br />
Widden P, Parkinson D. 1973. Fungi from Canadian<br />
coniferous forest soils. Can J Bot 51:2275–2290.<br />
Wildman HG, Parkinson D. 1978. Micr<strong>of</strong>ungal succession<br />
on living leaves <strong>of</strong> Populus tremuloides. Can J Bot 57:<br />
2800–2811.<br />
Wilson D, Carroll GC. 1994. Infection studies <strong>of</strong> Discula<br />
quercina, an endophyte <strong>of</strong> Quercus garryana. <strong>Mycologia</strong><br />
86:635–647.