14: Substituent Effects
14: Substituent Effects
14: Substituent Effects
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
(9/94)(11,12/96)(11,12/04,01/05) Neuman Chapter <strong>14</strong><br />
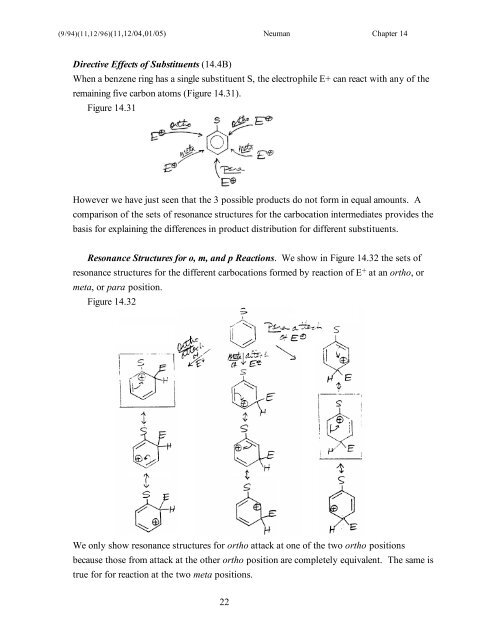
Directive <strong>Effects</strong> of <strong>Substituent</strong>s (<strong>14</strong>.4B)<br />
When a benzene ring has a single substituent S, the electrophile E+ can react with any of the<br />
remaining five carbon atoms (Figure <strong>14</strong>.31).<br />
Figure <strong>14</strong>.31<br />
However we have just seen that the 3 possible products do not form in equal amounts. A<br />
comparison of the sets of resonance structures for the carbocation intermediates provides the<br />
basis for explaining the differences in product distribution for different substituents.<br />
Resonance Structures for o, m, and p Reactions. We show in Figure <strong>14</strong>.32 the sets of<br />
resonance structures for the different carbocations formed by reaction of E + at an ortho, or<br />
meta, or para position.<br />
Figure <strong>14</strong>.32<br />
We only show resonance structures for ortho attack at one of the two ortho positions<br />
because those from attack at the other ortho position are completely equivalent. The same is<br />
true for for reaction at the two meta positions.<br />
22