14: Substituent Effects
14: Substituent Effects
14: Substituent Effects
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
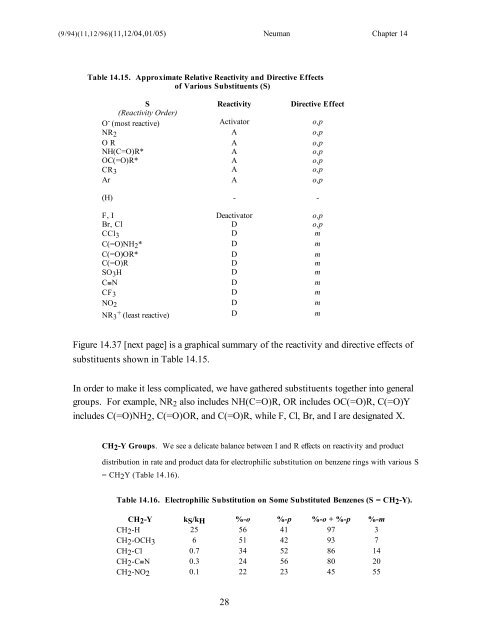
(9/94)(11,12/96)(11,12/04,01/05) Neuman Chapter <strong>14</strong><br />
Table <strong>14</strong>.15. Approximate Relative Reactivity and Directive <strong>Effects</strong><br />
of Various <strong>Substituent</strong>s (S)<br />
S Reactivity Directive Effect<br />
(Reactivity Order)<br />
O - (most reactive) Activator o,p<br />
NR2 A o,p<br />
O R A o,p<br />
NH(C=O)R* A o,p<br />
OC(=O)R* A o,p<br />
CR3 A o,p<br />
Ar A o,p<br />
(H) - -<br />
F, I Deactivator o,p<br />
Br, Cl D o,p<br />
CCl3 D m<br />
C(=O)NH2* D m<br />
C(=O)OR* D m<br />
C(=O)R D m<br />
SO3H D m<br />
C≡N D m<br />
CF3 D m<br />
NO2 D m<br />
NR3 + (least reactive) D m<br />
Figure <strong>14</strong>.37 [next page] is a graphical summary of the reactivity and directive effects of<br />
substituents shown in Table <strong>14</strong>.15.<br />
In order to make it less complicated, we have gathered substituents together into general<br />
groups. For example, NR2 also includes NH(C=O)R, OR includes OC(=O)R, C(=O)Y<br />
includes C(=O)NH2, C(=O)OR, and C(=O)R, while F, Cl, Br, and I are designated X.<br />
CH2-Y Groups. We see a delicate balance between I and R effects on reactivity and product<br />
distribution in rate and product data for electrophilic substitution on benzene rings with various S<br />
= CH2Y (Table <strong>14</strong>.16).<br />
Table <strong>14</strong>.16. Electrophilic Substitution on Some Substituted Benzenes (S = CH2-Y).<br />
CH2-Y kS/kH %-o %-p %-o + %-p %-m<br />
CH2-H 25 56 41 97 3<br />
CH2-OCH3 6 51 42 93 7<br />
CH2-Cl 0.7 34 52 86 <strong>14</strong><br />
CH2-C≡N 0.3 24 56 80 20<br />
CH2-NO2 0.1 22 23 45 55<br />
28