lecture890708 - Workspace
lecture890708 - Workspace
lecture890708 - Workspace
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
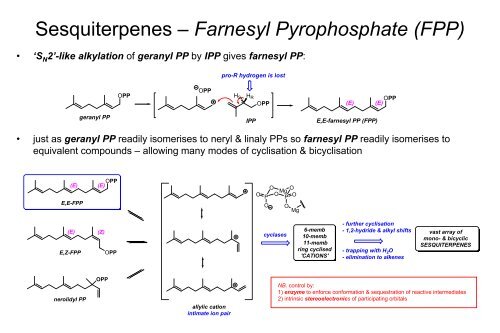
Sesquiterpenes – Farnesyl Pyrophosphate (FPP)<br />
• ‘S N2’-like alkylation of geranyl PP by IPP gives farnesyl PP:<br />
geranyl PP<br />
OPP<br />
pro-R hydrogen is lost<br />
• just as geranyl PP readily isomerises to neryl & linaly PPs so farnesyl PP readily isomerises to<br />
equivalent compounds – allowing many modes of cyclisation & bicyclisation<br />
E,Z-FPP<br />
nerolidyl PP<br />
(E) (E) OPP<br />
E,E-FPP<br />
(E)<br />
(Z)<br />
OPP<br />
OPP<br />
OPP<br />
allylic cation<br />
intimate ion pair<br />
H S<br />
H R<br />
IPP<br />
OPP<br />
O<br />
O<br />
P O O<br />
Mg<br />
P<br />
O<br />
O<br />
cyclases<br />
O<br />
Mg<br />
6-memb<br />
10-memb<br />
11-memb<br />
ring cyclised<br />
'CATIONS'<br />
(E)<br />
E,E-farnesyl PP (FPP)<br />
OPP<br />
(E)<br />
- further cyclisation<br />
- 1,2-hydride & alkyl shifts<br />
- trapping with H 2O<br />
- elimination to alkenes<br />
vast array of<br />
mono- & bicyclic<br />
SESQUITERPENES<br />
NB. control by:<br />
1) enzyme to enforce conformation & sequestration of reactive intermediates<br />
2) intrinsic stereoelectronics of participating orbitals