lecture890708 - Workspace
lecture890708 - Workspace
lecture890708 - Workspace
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
H<br />
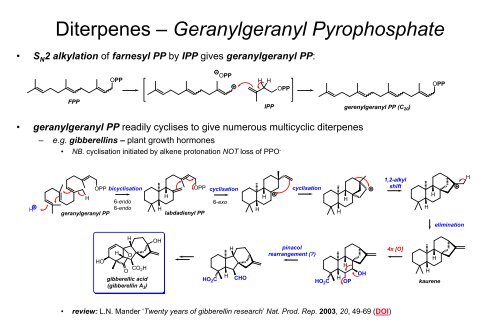
Diterpenes – Geranylgeranyl Pyrophosphate<br />
• S N2 alkylation of farnesyl PP by IPP gives geranylgeranyl PP:<br />
FPP<br />
OPP bicyclisation OPP cyclisation cyclisation<br />
H<br />
6-endo<br />
H<br />
6-exo<br />
H<br />
geranylgeranyl PP<br />
6-endo H<br />
labdadienyl PP<br />
H<br />
HO<br />
OPP<br />
• geranylgeranyl PP readily cyclises to give numerous multicyclic diterpenes<br />
– e.g. gibberellins – plant growth hormones<br />
H<br />
H<br />
O<br />
CO<br />
O 2H<br />
gibberellic acid<br />
(gibberellin A3) OH<br />
OPP<br />
• NB. cyclisation initiated by alkene protonation NOT loss of PPO -<br />
H<br />
HO 2C H CHO<br />
pinacol<br />
rearrangement (?)<br />
HO 2C<br />
H<br />
H<br />
H<br />
H<br />
OP<br />
• review: L.N. Mander ‘Twenty years of gibberellin research’ Nat. Prod. Rep. 2003, 20, 49-69 (DOI)<br />
H<br />
H<br />
IPP<br />
OPP<br />
gerenylgeranyl PP (C 20)<br />
OH<br />
1,2-alkyl<br />
shift<br />
4x [O]<br />
H<br />
H<br />
OPP<br />
H<br />
H<br />
kaurene<br />
elimination<br />
H