Mosher Ester Analysis - Department of Chemistry and Physics
Mosher Ester Analysis - Department of Chemistry and Physics
Mosher Ester Analysis - Department of Chemistry and Physics
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
called kinetic resolution. Usually this is avoided by using an excess <strong>of</strong> the enantiopure<br />
reactant <strong>and</strong> using reactions that are fast <strong>and</strong> irreversible.<br />
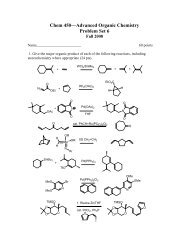
In this lab, you will prepare a chiral ester <strong>of</strong> a chiral secondary alcohol, 1phenylethanol.<br />
The ester is derived from the chiral carboxlic acid -methoxy-trifluoromethylphenylacetic<br />
acid (MTPA, <strong>Mosher</strong>’s acid), shown below.<br />
F3C<br />
MTPA<br />
Diastereomers, unlike enantiomers, have distinguishable physical <strong>and</strong> spectral<br />
properties because they are not mirror images. Diastereomers have two (or more) chiral<br />
centers. The added chiral center(s) can be the same or opposite configuration as that <strong>of</strong><br />
the original molecule. The matched <strong>and</strong> mismatched diastereomers will have different<br />
spectra <strong>and</strong> can thus be distinguished as shown below for one <strong>of</strong> the enantiomers <strong>of</strong><br />
MTPA <strong>and</strong> scalemic 1-phenylethanol.<br />
F3C<br />
O<br />
OCH 3<br />
OH<br />
+<br />
OH<br />
CH 3<br />
single enantiomer mixture <strong>of</strong> enantiomers<br />
mixture <strong>of</strong> diastereomers<br />
MTPA is a useful chiral acid for analyzing enantiomeric excess for several<br />
reasons. First, both enantiomers are available in high enantiomeric purity. Second, the<br />
chiral center is adjacent to the carboxyl bringing it in close proximity to the chiral center<br />
<strong>of</strong> the alcohol when the ester is prepared. Also, the chiral center is not able to be<br />
racemized as it is quaternary <strong>and</strong> thus contains no acidic protons. Finally, the presence <strong>of</strong><br />
the trifluoromethyl group allows for clean analysis <strong>of</strong> enantiomeric excess by 19 F NMR,<br />
which is uncomplicated by signals from the chiral alcohol, unlike the 1 H NMR spectrum.<br />
However, 1 H NMR can also be used if the signals are sufficiently resolved. This also<br />
allows for assignment <strong>of</strong> configuration based on a preferred conformation <strong>of</strong> the ester. In<br />
this experiment, you will team up with a partner. You will be given a sample <strong>of</strong> 1phenylethanol<br />
<strong>of</strong> unknown enantiomeric composition. One <strong>of</strong> you will prepare the (R)-<br />
(+)-MTPA ester <strong>of</strong> your 1-phenylethanol sample <strong>and</strong> the other will prepare the (S)-(-)-<br />
MTPA ester <strong>of</strong> the sample. You will then compare your 19 F NMR <strong>and</strong> 1 H NMR data <strong>and</strong><br />
determine both the enantiomeric excess <strong>and</strong> the major enantiomer present according to<br />
the published methods by <strong>Mosher</strong>.<br />
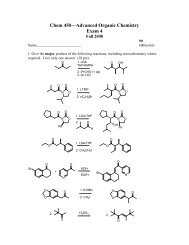
We will prepare our esters using N,N’-dicyclohexylcarbodiimide (DCC) to<br />
activate the acid toward substitution <strong>and</strong> 4-dimethylaminopyridine (DMAP) as a catalyst.<br />
*<br />
O<br />
OCH 3<br />
OH<br />
F3C<br />
F3C<br />
O<br />
OCH 3<br />
O<br />
+<br />
OCH 3<br />
H<br />
O<br />
H<br />
O<br />
CH 3<br />
CH 3