Chem 450—Advanced Organic Chemistry Exam 4
Chem 450—Advanced Organic Chemistry Exam 4
Chem 450—Advanced Organic Chemistry Exam 4
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Chem</strong> <strong>450—Advanced</strong> <strong>Organic</strong> <strong>Chem</strong>istry<br />
<strong>Exam</strong> 4<br />
Fall 2008<br />
90<br />
Name________________________ 100 points<br />
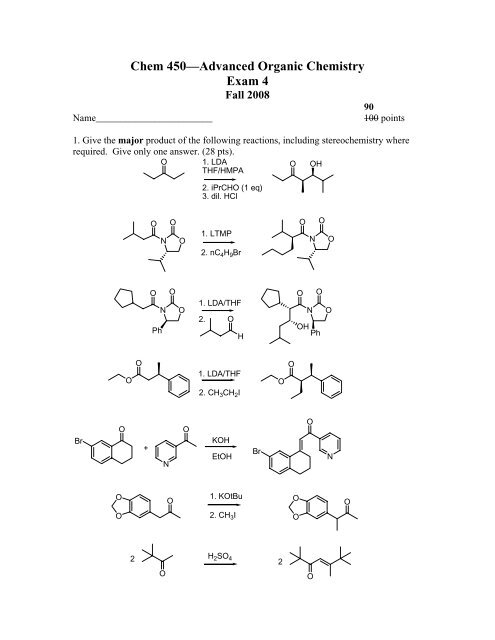
1. Give the major product of the following reactions, including stereochemistry where<br />
required. Give only one answer. (28 pts).<br />
O 1. LDA<br />
O OH<br />
THF/HMPA<br />
Br<br />
O<br />
O<br />
O<br />
O<br />
2<br />
O<br />
+<br />
O<br />
O<br />
Ph<br />
N<br />
N<br />
O<br />
N<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
2. iPrCHO (1 eq)<br />
3. dil. HCl<br />
1. LTMP<br />
2. nC 4H 9Br<br />
1. LDA/THF<br />
2.<br />
H<br />
1. LDA/THF<br />
2. CH 3CH 2I<br />
KOH<br />
O<br />
EtOH<br />
1. KOtBu<br />
2. CH 3I<br />
H 2SO 4<br />
Br<br />
O<br />
2<br />
O<br />
O<br />
O<br />
N<br />
N<br />
O<br />
O<br />
OH<br />
Ph<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
N<br />
O
2. Give the structure major enolate formed under the following conditions. Give only<br />
one answer. (12 pts).<br />
O<br />
O<br />
O<br />
O<br />
N<br />
O<br />
O<br />
O<br />
LDA<br />
THF/HMPA<br />
LDA<br />
THF<br />
KHMDS<br />
THF<br />
LTMP<br />
THF<br />
OLi<br />
Li<br />
O O<br />
3. Give the reagents required for the following transformations (9 pts).<br />
O<br />
O<br />
O<br />
O<br />
H<br />
O<br />
Et 3N<br />
THF<br />
1. LDA/HMPA<br />
2. PhCH 2CHO<br />
3. Dilute H 3O +<br />
KOH<br />
EtOH, ∆<br />
O<br />
O<br />
OLi<br />
O<br />
OH<br />
N<br />
OK<br />
NO 2<br />
O OH<br />
racemate<br />
4. What is a “directed” aldol reaction (3 points)?<br />
An aldol in which the enolate is preformed. Thus only the preformed enolate is able<br />
to react with the carbonyl compound, forcing condensation to occur in only one<br />
direction.<br />
O<br />
O
5. Give all possible products of the following reaction and indicate the major product (10<br />
pts).<br />
2<br />
O NaOH<br />
CF 3<br />
EtOH, reflux<br />
F 3C<br />
6. Give a transition state explaining the stereochemistry of the enolate below (4 points).<br />
O<br />
O<br />
LDA<br />
THF<br />
OH<br />
N<br />
H<br />
O<br />
Li<br />
O<br />
O<br />
THF<br />
CF 3<br />
CF 3<br />
THF<br />
7. Explain the stereochemical outcome of the following reaction (4 points).<br />
H<br />
O<br />
O<br />
H<br />
O<br />
1. LDA/THF<br />
2. BnBr<br />
The enolate is attacked from the convex face opposite the aromatic ring as the H is<br />
smaller. (The approach will also depend on the configuration next to the oxygen,<br />
though this was not specified.)<br />
+<br />
and<br />
+<br />
Bn<br />
H<br />
OLi<br />
F 3C<br />
O<br />
O<br />
O<br />
OH<br />
O<br />
O<br />
CF 3<br />
CF 3
8. Give structures illustrating why LDA alone promotes formation of the E-enolate, while<br />
addition of HMPA promotes the formation of Z-enolates (8 points)?<br />
Open TS Li does not bridge<br />
Closed TS - Cyclic<br />
H<br />
O<br />
H<br />
N<br />
O Li<br />
THF<br />
THF<br />
9. Explain why the following reaction does not produce the desired product and suggest a<br />
likely structure for the major product obtained (6 points).<br />
O<br />
+<br />
O<br />
NaOH<br />
CH3 O<br />
H3C CH3 H3C H<br />
EtOH H3C H<br />
O<br />
H<br />
OH O<br />
OH O<br />
H3C H<br />
or<br />
H3C CH3 Aldehyde is much more reactive. Also, no heat so no elimination.<br />
10. The following compound was prepared using a Robinson annulation. Give the<br />
structures of the starting materials (6 points).<br />
O<br />
+<br />
O<br />
KOH<br />
EtOH<br />
O<br />
N<br />
H<br />
O
Extra Credit 5 points: Give a step by step mechanism for the product below.<br />
O<br />
H<br />
H<br />
O<br />
+ CH 3NO 2<br />
NaOH + CH3NO2 H2C<br />
O<br />
N<br />
O<br />
HO<br />
O<br />
N<br />
H<br />
O<br />
O<br />
H2C N<br />
O<br />
NO 2<br />
HO O<br />
H<br />
Na +<br />
O<br />
O<br />
H<br />
HO<br />
H<br />
cat. NaOH<br />
O<br />
O<br />
N H<br />
O<br />
EtOH<br />
+ Na +<br />
H<br />
O<br />
H2C N<br />
O<br />
O<br />
NO 2<br />
HO OH<br />
O<br />
H2C O<br />
N<br />
+ Na<br />
O<br />
+ + H2O O<br />
N H<br />
H<br />
O<br />
HO<br />
Na +<br />
O<br />
N<br />
H<br />
O<br />
NO2 + H2O HO OH<br />
+ NaOH<br />
H<br />
H<br />
O<br />
O