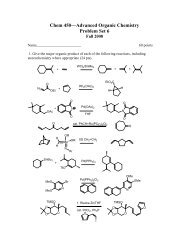
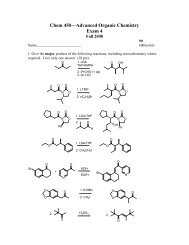
Mosher Ester Analysis - Department of Chemistry and Physics
Mosher Ester Analysis - Department of Chemistry and Physics
Mosher Ester Analysis - Department of Chemistry and Physics
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Mosher</strong> <strong>Analysis</strong> <strong>of</strong> Enantiomeric Purity <strong>and</strong> Assignment <strong>of</strong> Absolute Configuratoin<br />
by NMR spectroscopy.<br />
References:<br />
Patterson, J.; Sigurdsson, S.T. J. Chem. Educ. 2005, 82, 1049-1050.<br />
Sullivan, G. R.; Dale, J.A.; <strong>Mosher</strong>, H.S. J. Am. Chem. Soc. 1973, 95, 512-519.<br />
Dale, J.A.; <strong>Mosher</strong>, H.S. J. Org. Chem. 1973, 38, 2143-2147.<br />
Prelab: Prepare your reagent table <strong>and</strong> procedure sheet, including the reaction. The<br />
reaction is described below. Have them ready to do the experiment when you come into<br />
the lab. You can find all <strong>of</strong> the reagents at www.sigmaaldrich.com. Also get NMR<br />
spectra for the starting materials that you can use to compare with your crude product.<br />
Background<br />
While we <strong>of</strong>ten discuss chiral compounds as single enantiomers, it is common to<br />
encounter these compounds as mixtures <strong>of</strong> enantiomers. This occurs both in the<br />
laboratory <strong>and</strong> in nature. In the laboratory, this most commonly arises from the use <strong>of</strong><br />
less than enantiopure starting materials or reagents during synthesis or by racemization<br />
during reactions. In nature, this can be due to competing enantomeric biosynthetic<br />
pathways <strong>and</strong> by enzymatic or chemical racemization. Mixtures <strong>of</strong> enantiomers can be<br />
racemic (50:50 mixture <strong>of</strong> enantiomers) or scalemic (any other proportion).<br />
The analysis <strong>of</strong> enantiomeric mixtures can be divided into two objectives. The<br />
first is the analysis <strong>of</strong> enantiomeric excess <strong>and</strong> the second is the establishment <strong>of</strong> absolute<br />
configuration. Enantiomeric excess is the amount <strong>of</strong> one enantiomer over the other <strong>and</strong> is<br />
usually expressed as a percentage value. Percent e.e. is calculated as:<br />
% e.e. = (100%)|R – S|<br />
R + S<br />
Establishment <strong>of</strong> configuration is simply determining which enantiomer is R <strong>and</strong><br />
which is S, <strong>and</strong> determination <strong>of</strong> which is in excess. The latter is a difficult task as,<br />
enantiomers are identical in all physical <strong>and</strong> spectral properties. Enantiomers do differ in<br />
the sign <strong>of</strong> their optical rotation <strong>and</strong> certain compounds can be distinguished by<br />
spectropolarimetry (the study <strong>of</strong> variation <strong>of</strong> rotation with wavelength, <strong>of</strong>ten referred to<br />
as circular dichroism (CD) or optical rotatory dispersion (ORD)). However, this<br />
approach is <strong>of</strong>ten limited to certain classes <strong>of</strong> compounds with large changes in their<br />
rotation <strong>and</strong> it is not generally applicable.<br />
In many cases both assignment <strong>of</strong> configuration <strong>and</strong> determination <strong>of</strong> e.e. can be<br />
achieved by reacting the molecule in question with a chiral compound <strong>of</strong> known absolute<br />
configuration <strong>and</strong> enantiomeric purity (preferably a single enantiomer). This produces a<br />
mixture <strong>of</strong> diastereomers. The requirements for such a method are that the chemistry<br />
involved does not cause the chiral centers <strong>of</strong> either <strong>of</strong> the reactants to racemize <strong>and</strong> that<br />
the reaction is quantitative in reaction with both enantiomers <strong>of</strong> the compound you are<br />
analyzing. That is, in some cases, one enantiomer reacts faster than the other. This is
called kinetic resolution. Usually this is avoided by using an excess <strong>of</strong> the enantiopure<br />
reactant <strong>and</strong> using reactions that are fast <strong>and</strong> irreversible.<br />
In this lab, you will prepare a chiral ester <strong>of</strong> a chiral secondary alcohol, 1phenylethanol.<br />
The ester is derived from the chiral carboxlic acid -methoxy-trifluoromethylphenylacetic<br />
acid (MTPA, <strong>Mosher</strong>’s acid), shown below.<br />
F3C<br />
MTPA<br />
Diastereomers, unlike enantiomers, have distinguishable physical <strong>and</strong> spectral<br />
properties because they are not mirror images. Diastereomers have two (or more) chiral<br />
centers. The added chiral center(s) can be the same or opposite configuration as that <strong>of</strong><br />
the original molecule. The matched <strong>and</strong> mismatched diastereomers will have different<br />
spectra <strong>and</strong> can thus be distinguished as shown below for one <strong>of</strong> the enantiomers <strong>of</strong><br />
MTPA <strong>and</strong> scalemic 1-phenylethanol.<br />
F3C<br />
O<br />
OCH 3<br />
OH<br />
+<br />
OH<br />
CH 3<br />
single enantiomer mixture <strong>of</strong> enantiomers<br />
mixture <strong>of</strong> diastereomers<br />
MTPA is a useful chiral acid for analyzing enantiomeric excess for several<br />
reasons. First, both enantiomers are available in high enantiomeric purity. Second, the<br />
chiral center is adjacent to the carboxyl bringing it in close proximity to the chiral center<br />
<strong>of</strong> the alcohol when the ester is prepared. Also, the chiral center is not able to be<br />
racemized as it is quaternary <strong>and</strong> thus contains no acidic protons. Finally, the presence <strong>of</strong><br />
the trifluoromethyl group allows for clean analysis <strong>of</strong> enantiomeric excess by 19 F NMR,<br />
which is uncomplicated by signals from the chiral alcohol, unlike the 1 H NMR spectrum.<br />
However, 1 H NMR can also be used if the signals are sufficiently resolved. This also<br />
allows for assignment <strong>of</strong> configuration based on a preferred conformation <strong>of</strong> the ester. In<br />
this experiment, you will team up with a partner. You will be given a sample <strong>of</strong> 1phenylethanol<br />
<strong>of</strong> unknown enantiomeric composition. One <strong>of</strong> you will prepare the (R)-<br />
(+)-MTPA ester <strong>of</strong> your 1-phenylethanol sample <strong>and</strong> the other will prepare the (S)-(-)-<br />
MTPA ester <strong>of</strong> the sample. You will then compare your 19 F NMR <strong>and</strong> 1 H NMR data <strong>and</strong><br />
determine both the enantiomeric excess <strong>and</strong> the major enantiomer present according to<br />
the published methods by <strong>Mosher</strong>.<br />
We will prepare our esters using N,N’-dicyclohexylcarbodiimide (DCC) to<br />
activate the acid toward substitution <strong>and</strong> 4-dimethylaminopyridine (DMAP) as a catalyst.<br />
*<br />
O<br />
OCH 3<br />
OH<br />
F3C<br />
F3C<br />
O<br />
OCH 3<br />
O<br />
+<br />
OCH 3<br />
H<br />
O<br />
H<br />
O<br />
CH 3<br />
CH 3
This is an exceptionally mild method <strong>of</strong> carboxyl activation <strong>and</strong> is used extensively for<br />
the synthesis <strong>of</strong> both esters an amides, particularly for peptide synthesis.<br />
O<br />
R OH<br />
R'OH, DCC, DMAP<br />
CH 2Cl 2<br />
O<br />
R OR'<br />
The reaction proceeds in two steps. First, the the acid reacts with DCC, forming<br />
an O-acylisourea. This species is similar to an anhydride <strong>and</strong> the carbonyl is highly<br />
susceptible to substitution. In the second step, the alcohol attacks the carbonyl to produce<br />
the ester <strong>and</strong> eliminates dicyclohexylurea as shown below.<br />
Step 1.<br />
O<br />
R O<br />
Step 2.<br />
O<br />
N<br />
c-Hx<br />
c-Hx<br />
R O N<br />
H<br />
O-acylisourea<br />
(similar to an anhydride)<br />
O<br />
H<br />
+<br />
N C N<br />
Dicyclohexylcarbodiimide<br />
(DCC)<br />
O<br />
HN<br />
c-Hx<br />
+<br />
c-Hx<br />
R OR'<br />
O N<br />
H<br />
Dicyclohexylurea (DCU)<br />
N<br />
c-Hx<br />
O<br />
R O<br />
O<br />
R O<br />
R' O H +<br />
c-Hx<br />
R' O +<br />
R O N<br />
H<br />
+<br />
+<br />
O<br />
H<br />
R O<br />
OR'<br />
H<br />
N C N<br />
H<br />
N C N<br />
H-bond<br />
O<br />
R O<br />
While this process can proceed by itself or with added bases such as<br />
triethylamine, it is somewhat sluggish. In order to speed the process, a nucleophilic<br />
N<br />
c-Hx<br />
N<br />
H<br />
H<br />
N<br />
c-Hx<br />
c-Hx<br />
N<br />
H<br />
c-Hx
catalyst, 4-dimethylaminopyridine (DMAP) is used. DMAP facilitates the process both<br />
by acting as a base <strong>and</strong> by forming activated acylpyridinium derivatives (below) from<br />
DCC <strong>and</strong> from the O-acylisourea. These are much more reactive as the pyridine is an<br />
excellent leaving group. This accelerates the reaction severalfold over the uncatalyzed<br />
reaction.<br />
H 3C<br />
N<br />
CH 3<br />
N<br />
N<br />
c-Hx<br />
N<br />
H<br />
c-Hx<br />
Procedure<br />
Wear gloves. DCC <strong>and</strong> DMAP are very toxic.<br />
You will be provided with a culture tube containing a scalemic solution <strong>of</strong> 6.0 L<br />
<strong>of</strong> 1-phenylethanol in 1 mL <strong>of</strong> dry CH2Cl2. To this add a stir bar <strong>and</strong> 13 mg <strong>of</strong> (R)-(+)- or<br />
(S)-(-)-MTPA as directed by your instructor. Be sure to record which enantiomer <strong>of</strong><br />
MTPA you use. Also be sure to cap the tube quickly to minimize any moisture entry<br />
after each addition <strong>of</strong> reagent. A nitrogen balloon <strong>and</strong> septum can be used if you wish.<br />
Cool the flask in an ice bath <strong>and</strong> add 12 mg <strong>of</strong> DCC <strong>and</strong> a spatula point (~1-2 mg) <strong>of</strong><br />
DMAP in sequence. Add the DCC to the reaction as soon as it is weighed as it tends to<br />
pick up moisture in the air. Stir the solution for 5 minutes in the ice bath, <strong>and</strong> then 25<br />
minutes at room temperature, during which time dicyclohexylurea will precipitate.<br />
While you are waiting on the reaction to complete, experiment with different TLC<br />
systems to determine if your reaction has finished. Spot 1-phenylethanol, DCC, DMAP,<br />
MTPA, <strong>and</strong> your reaction separately on the TLC plate <strong>and</strong> develop. Visualize the plate<br />
with UV light <strong>and</strong> circle the spots with a pencil. Begin with 1:1 hexane-ethyl acetate <strong>and</strong><br />
evaluate several mixtures until you find one that separates all <strong>of</strong> the components well,<br />
especially the product. This mixture will be used for preparative TLC to purify the<br />
product.<br />
Also while you are waiting on the reaction to complete, prepare two large test<br />
tubes for exraction <strong>of</strong> the reaction mixture. To one tube add 2 mL <strong>of</strong> 5% NaHCO3 <strong>and</strong> to<br />
the second add 2 mL <strong>of</strong> saturated NaHCO3.<br />
When the reaction is judged to be complete or 30 minutes passed, add 2 drops <strong>of</strong><br />
water <strong>and</strong> stir an additional 10 minutes. Then, add one mL <strong>of</strong> CH2Cl2 <strong>and</strong> 2 mL <strong>of</strong> 5%<br />
acetic acid to the culture tube <strong>and</strong> swirl vigorously. Using a pipet, transfer the organic<br />
layer to the test tube containing 5% NaHCO3 <strong>and</strong> swirl as before. Repeat this for the tube<br />
containing saturated NaHCO3. Prepare a pipet drying column plugged with glass wool<br />
<strong>and</strong> filled with ~3 cm <strong>of</strong> anhydrous Na2SO4. Clamp the tube in place <strong>and</strong> place a tared 10<br />
mL round bottom flask below to collect the eluent. Add the organic phase from the last<br />
tube <strong>and</strong> allow it to pass through the column <strong>and</strong> collect in the flask. Once all <strong>of</strong> the<br />
organic phase has been added <strong>and</strong> the top <strong>of</strong> the liquid reaches the Na2SO4, wash the<br />
column twice with 2 mL CH2Cl2. Remove the solvent by rotary evaporation <strong>and</strong> remove<br />
any traces under high vacuum for no more than a minute or two (The instructor will show<br />
R<br />
O<br />
N<br />
N<br />
CH 3<br />
CH 3
you how to do this). Obtain a crude yield, dissolve the product in CDCl3 <strong>and</strong> obtain a 1 H<br />
NMR spectrum <strong>of</strong> the crude ester.<br />
Preparative Thin-Layer Chromatography<br />
The <strong>Mosher</strong> ester will be purified by preparative thin-layer chromatography. This<br />
is essentially the same as what you use for monitoring your reactions, only on larger<br />
scale. We will use a large developing tank, <strong>and</strong> large, thickly coated plates. The plates<br />
we are using are capable <strong>of</strong> separating around 10-20 mg, depending on separation<br />
efficiency. Unlike flash chromatography, no specific Rf value is required. Simply<br />
identify a solvent system which will adequately separate your compounds <strong>and</strong> develop<br />
the plate. The technique does have limitations, however. The large surface area <strong>of</strong> the<br />
silica, combined with its mildly acidic nature tend to promote air oxidation <strong>of</strong> sensitive<br />
functionalities such as aldehydes, amines, <strong>and</strong> reactive double bonds. Also, the method is<br />
rather expensive to perform on other than small scale separations. Nonetheless, it is <strong>of</strong><br />
great utility in difficult separations <strong>of</strong> small amounts <strong>of</strong> material, such as extracts <strong>of</strong><br />
natural products <strong>and</strong> separation <strong>of</strong> diastereomers. In the second part <strong>of</strong> this experiment,<br />
you will separate the products <strong>of</strong> your <strong>Mosher</strong> ester reaction by preparative TLC <strong>and</strong><br />
characterize your product by 1 H <strong>and</strong> 19 FNMR.<br />
Preparative TLC Procedures<br />
1. Set up TLC Developing tank.<br />
a. Examine TLC’s in various solvent systems (TLC hood) if you have not yet<br />
determined an optimal composition.<br />
b. Dissolve your sample in 1.0 mL CH2Cl2 or use the NMR sample directly.<br />
c. Perform a TLC on your material using that system. (Don’t forget to cospot<br />
with your starting material).<br />
d. Make up 100 mL <strong>of</strong> your chosen solvent system <strong>and</strong> put it in the tank.<br />
2. Prepare your TLC plate.<br />
a. Set up the solvent streaker (Have the instructor help you.)<br />
b. Draw a pencil line about 1” from the bottom <strong>of</strong> the plate.<br />
c. Set the streaker up so that the tip <strong>of</strong> the capillary traverses this line as you<br />
draw it along the benchtop.<br />
d. Fill the streaker with your solution. The reservoir will only hold around<br />
0.25-0.5 mL.<br />
e. Streak the plate on the line you drew. Try to keep the thickness <strong>of</strong> your<br />
b<strong>and</strong> between 1-3mm. Wave your h<strong>and</strong> over the plate to assist drying.<br />
f. Refill the reservoir again <strong>and</strong> repeat.<br />
g. Clean the reservoir with acetone. Dry it under a nitrogen flow. Give it to<br />
the next person to use. Be gentle with the streaker. We only have one <strong>and</strong><br />
if broken, it will end our experiment.<br />
3. Develop your plate.<br />
a. Place your plate in the developing tank.<br />
b. Develop the plate until the solvent front reaches within one inch <strong>of</strong> the top.<br />
This will take about an hour. Be sure to check the plate every 15 minutes<br />
to see its progress.
c. Remove the plate from the tank <strong>and</strong> mark the solvent front with a pencil.<br />
d. Wait for the plate to dry. You can assist this by fanning the plate with a<br />
folder or using a flow <strong>of</strong> dry nitrogen. While you are waiting, you should<br />
be cleaning glassware or something else productive. Do not wait an<br />
excessive amount <strong>of</strong> time. Many compounds are sensitive to oxidation on<br />
a TLC plate as the surface area is very large.<br />
4. Isolate the product.<br />
a. When your plate is dry enough, visualize the b<strong>and</strong>s using UV light. Mark<br />
them lightly with a pencil.<br />
b. Using an exacto knife or the flat edge <strong>of</strong> a spatula, scrape the b<strong>and</strong>s <strong>of</strong>f<br />
onto a lengthwise folded piece <strong>of</strong> clean, white paper.<br />
c. Prepare a vacuum filtration using a fritted glass funnel as shown below.<br />
d. Place the scrapings into a fritted glass funnel <strong>and</strong> press them into a pad.<br />
e. Wash the compound <strong>of</strong>f the silica into a round bottomed flask using 3 x 5<br />
mL <strong>of</strong> TLC solvent.<br />
f. Remove the solvent by rotary evaporation <strong>and</strong> determine the mass <strong>of</strong> your<br />
product. While this is occurring have your sample analyzed by chiral GC<br />
or be cleaning your glassware.<br />
5. Characterize your product by 1 H <strong>and</strong> 19 FNMR.<br />
Since one member <strong>of</strong> your team will prepare the R-MTPA ester <strong>and</strong> the other will<br />
prepare the S-MTPA ester, compare your results <strong>and</strong> assign the stereochemistry <strong>of</strong> the<br />
alcohol <strong>and</strong> the % e.e. according to the two <strong>Mosher</strong> references.<br />
Questions:<br />
1. Compare the % e.e. based on the integration <strong>of</strong> the 1 H <strong>and</strong> the 19 F spectra <strong>of</strong> the<br />
<strong>Mosher</strong> ester you made. For 1 H, calculate for all resonances that are sufficiently<br />
resolved to integrate reliably. Do they agree? If not, suggest a reason for the<br />
discrepancy.<br />
2. Calculate the % e.e. from your crude product <strong>and</strong> compare with that from the<br />
purified sample. Do they agree? If not, suggest a reason for the discrepancy.<br />
3. Compare the % e.e. you obtained to that obtained by your partner. Do they agree?<br />
If not, suggest a reason for the discrepancy.<br />
4. Suggest two alternate methods for determining the % e.e. <strong>of</strong> your alcohol.
Plate<br />
20 cm x 20 cm<br />
2.5 mm layer<br />
thickness<br />
Bench Guide/<br />
Reservoir Holder<br />
Streak line<br />
~1" from bottom<br />
1-3mm thickness<br />
Glass Plate Cover<br />
Developing chamber<br />
Solvent<br />
~3/4" from bottom<br />
About 100 mL<br />
TLC Plate Streaker<br />
Bench<br />
Preparative TLC Plate<br />
Development<br />
Reservoir<br />
Capillary<br />
TLC Plate<br />
to aspirator<br />
25 mL<br />
Claisen<br />
head