Ph. D. THESIS 2009
Ph. D. THESIS 2009
Ph. D. THESIS 2009
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
(mol·l -1 )<br />
syn 8.8 x10 -3 1.4373 5.10 3.549<br />
anti 8.8 x10 -3 0.69574 3.774 5.425<br />
In equations (1) and (2) k1 and k-1 are the forward and reverse reaction rate<br />
constants, K is the equilibrium constant, xe is the initial concentration of the<br />
starting isomer, x is the concentration of the same isomer at the “t” time.<br />
2.3.3. Chiral HPLC studies<br />
In order to establish the chiral behavior of syn and anti isomers of compound<br />
5, already separated by flash chromatography, a mixture of these isomers was<br />
subjected to HPLC separation using a chiral stationary phase.<br />
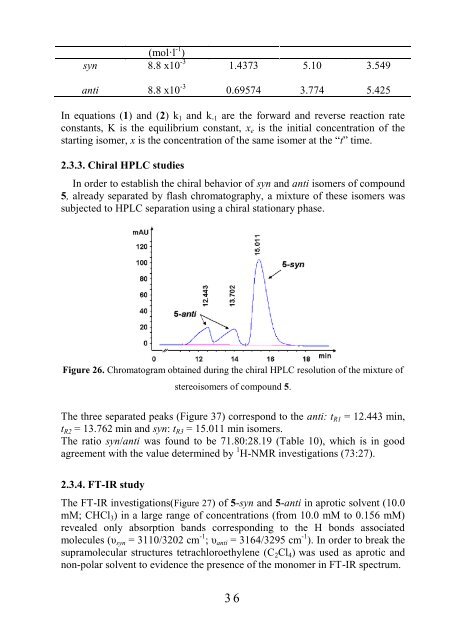
Figure 26. Chromatogram obtained during the chiral HPLC resolution of the mixture of<br />
stereoisomers of compound 5.<br />
The three separated peaks (Figure 37) correspond to the anti: tR1 = 12.443 min,<br />
tR2 = 13.762 min and syn: tR3 = 15.011 min isomers.<br />
The ratio syn/anti was found to be 71.80:28.19 (Table 10), which is in good<br />
agreement with the value determined by 1 H-NMR investigations (73:27).<br />
2.3.4. FT-IR study<br />
The FT-IR investigations(Figure 27) of 5-syn and 5-anti in aprotic solvent (10.0<br />
mM; CHCl3) in a large range of concentrations (from 10.0 mM to 0.156 mM)<br />
revealed only absorption bands corresponding to the H bonds associated<br />
molecules (υsyn = 3110/3202 cm -1 ; υanti = 3164/3295 cm -1 ). In order to break the<br />
supramolecular structures tetrachloroethylene (C2Cl4) was used as aprotic and<br />
non-polar solvent to evidence the presence of the monomer in FT-IR spectrum.<br />
36