Introduction: Heather Moss, MD, PhD (Attending) - University of ...
Introduction: Heather Moss, MD, PhD (Attending) - University of ...
Introduction: Heather Moss, MD, PhD (Attending) - University of ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Illinois Eye and Ear Infi rmary<br />
UIC Department <strong>of</strong> Ophthalmology & Visual Sciences<br />
<strong>Introduction</strong>: <strong>Heather</strong> <strong>Moss</strong>, <strong>MD</strong>, <strong>PhD</strong> (<strong>Attending</strong>)<br />
his Grand Rounds issue presents several interesting cases encountered by the<br />
T Neuro-Ophthalmology service.<br />
Edited by Rohan Shah, <strong>MD</strong> and Elizabeth Grace, <strong>MD</strong>. Photos by Mark Janowicz (unless otherwise noted). All photos are property <strong>of</strong> UIC.<br />
© Copyright 2012 by the Department <strong>of</strong> Ophthalmology, <strong>University</strong> <strong>of</strong> Illinois College <strong>of</strong> Medicine. All rights reserved. Cases presented in this issue are from<br />
UI Ophthalmology Grand Rounds from February 1, 2012.<br />
Compressive Optic Neuropathy: Kevin Patel, <strong>MD</strong> (Resident)<br />
A 50 year-old Caucasian male referred from<br />
the UIC oculoplastics clinic for further evaluation<br />
<strong>of</strong> diplopia. Symptoms began 1 year prior<br />
to presentation when the patient started having<br />
swelling <strong>of</strong> both eyes, OD worse than OS. His<br />
diplopia is worst in up, down, and left gaze.<br />
He also complains <strong>of</strong> blurred vision OD for 1<br />
month which is most pronounced centrally.<br />
The patient has a history <strong>of</strong> multiple sclerosis<br />
and his ocular symptoms were first attributed<br />
to this. However, an MRI was performed which<br />
showed no active signs <strong>of</strong> MS. IV steroids were used to treat the swelling<br />
and diplopia, which caused some improvement in symptoms. Over<br />
the past summer, the diplopia worsened and patient was started on<br />
oral prednisone. There was no improvement in symptoms, however,<br />
when he stopped the steroids, he had acute worsening <strong>of</strong> swelling<br />
most prominent in the right infraorbital region. He restarted oral prednisone<br />
at a higher dose (50 mg) with some mild improvement. The week<br />
prior in the oculoplastics clinic his visual acuity OD was 20/30 and the<br />
patient had mild disc hyperemia with nasal disc edema OD.<br />
Past medical history is significant for multiple sclerosis and hyperthyroidism.<br />
His medications include prednisone, Lipitor, methimazole,<br />
citalopram, lansoprazole, ibupr<strong>of</strong>en, and betaseron. He uses artificial<br />
tears as needed. Social history is significant for occasional alcohol<br />
consumption and marijuana use.<br />
Neuro-Ophthalmology<br />
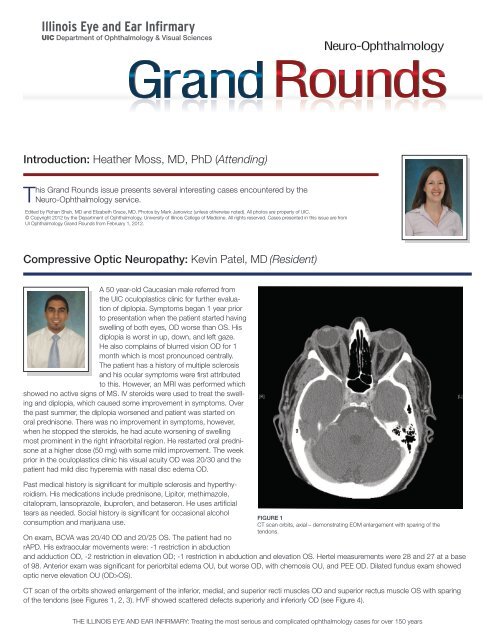
FIGURE 1<br />
CT scan orbits, axial – demonstrating EOM enlargement with sparing <strong>of</strong> the<br />
tendons.<br />
On exam, BCVA was 20/40 OD and 20/25 OS. The patient had no<br />
rAPD. His extraocular movements were: -1 restriction in abduction<br />
and adduction OD, -2 restriction in elevation OD; -1 restriction in abduction and elevation OS. Hertel measurements were 28 and 27 at a base<br />
<strong>of</strong> 98. Anterior exam was significant for periorbital edema OU, but worse OD, with chemosis OU, and PEE OD. Dilated fundus exam showed<br />
optic nerve elevation OU (OD>OS).<br />
CT scan <strong>of</strong> the orbits showed enlargement <strong>of</strong> the inferior, medial, and superior recti muscles OD and superior rectus muscle OS with sparing<br />
<strong>of</strong> the tendons (see Figures 1, 2, 3). HVF showed scattered defects superiorly and inferiorly OD (see Figure 4).<br />
THE ILLINOIS EYE AND EAR INFIRMARY: Treating the most serious and complicated ophthalmology cases for over 150 years
Compressive Optic Neuropathy: (Continued)<br />
The patient was diagnosed with thyroid eye disease with optic neuropathy OD. He was given IV solumedrol for 3 days, then transitioned to oral<br />
prednisone 80 mg daily. On follow-up, patient had much less swelling. BCVA had improved to 20/25-3 OD and 20/25 OS. Motility remained<br />
restricted. Dilated fundus exam showed minimal disc elevation OD and no disc elevation OS. A repeat HVF showed resolution <strong>of</strong> the scattered<br />
defects OD (see Figure 5).<br />
FIGURE 2<br />
CT scan orbits, coronal – demonstrating prominent EOM enlargement<br />
bilaterally.<br />
FIGURE 3<br />
CT scan orbits – EOM enlargement near apex, starting to encroach upon<br />
the right optic nerve.<br />
UIC Department <strong>of</strong> Ophthalmology and Visual Sciences, 1855 West Taylor Street, MC 648, Chicago, IL 60612<br />
FIGURE 4 (left)<br />
24-2 HVF OD – demonstrates<br />
scattered visual field defects<br />
superiorly and inferiorly.<br />
FIGURE 5 (right)<br />
24-2 HVF OD – 2 weeks after<br />
IV steroids, shows resolution <strong>of</strong><br />
previous visual field defects.
Compressive Optic Neuropathy: (Continued)<br />
DISCUSSION Thyroid eye disease (TED) is an autoimmune disease which is usually self-limited but can be progressive. It is most commonly<br />
associated with Graves’ disease and hyperthyroidism, but can occur in patients who are euthyroid or hypothyroid. TED incidence is<br />
four times higher in women than men. Diagnosis is usually made clinically but imaging with CT or MRI can be done. Classically this shows<br />
enlargement <strong>of</strong> the EOMs with sparing <strong>of</strong> the tendons. Common clinical characteristics consist <strong>of</strong> inflammation and congestion <strong>of</strong> EOMs,<br />
proptosis, eyelid retraction, lid lag, diplopia, lid edema, chemosis, exposure keratopathy, and possible optic neuropathy. Treatment starts<br />
with establishing a euthyroid state. The literature has shown that corticosteroids are the most effective initial treatment. There is no additional<br />
benefit from early surgical decompression. Intraorbital steroid injections are another possible form <strong>of</strong> treatment which has shown to be effective.<br />
Radiation therapy is another effective treatment option, especially in patients who have failed treatment with steroids.<br />
REFERENCES:<br />
Phillips ME, Marzban MM, Kathuria SS. Treatment <strong>of</strong> thyroid eye disease. Current Treatment Options in Neurology. 2010 Jan;12(1):64-9.<br />
Modjtahedi SP, Modjtahedi BS, Mansury AM, Selva D, Douglas RS, Goldberg RA, Leibovitch I. Pharmacological treatments for thyroid eye<br />
disease. Drugs. 2006;66(13):1685-700.<br />
Alkawas AA, Hussein AM, Shahien EA. Orbital steroid injection versus oral steroid therapy in management <strong>of</strong> thyroid-related ophthalmopathy.<br />
Clinical and Experimental Ophthalmology. 2010 Oct;38(7):692-7.<br />
Abboud M, Arabi A, Salti I, Geara F. Outcome <strong>of</strong> thyroid associated ophthalmopathy treated by radiation therapy. Radiation Oncology. 2011<br />
May;6:46.<br />
Acute Altitudinal Visual Field Defect with Optic Disc Edema: Sara Huh, <strong>MD</strong> (Resident)<br />
A 68 year old male presents with the chief complaint <strong>of</strong> left eye vision loss. His medical history includes shingles affecting<br />
V1, V2, and V3 branches <strong>of</strong> his left face 9 months prior to presentation at UIC. His shingles started with a rash on<br />
his skin, foreign body sensation in the left eye with photophobia. He was treated with oral valacyclovir for 30 days and<br />
Lotemax for the ocular involvement. With the resolution <strong>of</strong> the rash, he developed severe left sided facial pain and was<br />
treated for post-herpetic neuralgia with improvement <strong>of</strong> his symptoms. CTA and MRI <strong>of</strong> the brain were performed at<br />
the time showing non-specific periventricular T2 signaling, likely due to small vessel ischemic disease. 2 weeks prior to<br />
presentation, he noted a grey shade being pulled down over the superior portion <strong>of</strong> his left eye visual field, without ocular<br />
pain. He went to his local ophthalmologist where his visual acuity was 20/100 and examination showed a swollen left<br />
optic nerve. Visual field testing showed a dense superior altitudinal loss in the left eye (see Figure 1). His ophthalmologist<br />
ordered a few laboratory tests, including ESR, CRP and platelets which were normal, presumed to rule out giant cell<br />
arteritis. He was started on Valtrex 1 g daily. 1 week later his vision improved to 20/30 in the left eye.<br />
He has no known drug allergies, is currently on valacyclovir, gabapentin, and Lotemax eye drops to his left eye. He has a 20 pack year smoking<br />
history; however he quit 30 years ago and he consumes alcohol socially.<br />
On presentation to the neuro-ophthalmology clinic, his left eye visual acuity was 20/30 with full color plates. He had a Relative Afferent Pupillary<br />
Defect (rAPD) on the left and superior field defect on confrontational visual field testing. His intraocular pressures were within normal limits.<br />
His anterior segment examination was significant for blepharitis bilaterally, some conjunctival injection in the left eye with dense inferior punctate<br />
staining with fain subepithelial infiltrates. There was no evidence <strong>of</strong> corneal pseudodendrites.<br />
On dilated fundus examination, the vitreous was clear with small cup to disc ratio bilaterally. The left optic nerve was diffusely hyperemic and<br />
edematous. Otherwise his fundus examination was within normal limits.<br />
The question at this point becomes – was this an idiopathic non-arteritic anterior ischemic optic neuropathy or a zoster related optic neuropathy?<br />
The plan was to rule out potential vasculopathy due to Varicella zoster virus (VZV) as an etiology <strong>of</strong> ischemic optic nerve injury – MRI <strong>of</strong> the brain<br />
and orbits were ordered and a lumbar puncture performed for IgG and IgM antibodies and VZV DNA by PCR. He was treated with IV acyclovir for<br />
10 days followed by oral acyclovir and IV solumedrol.<br />
THE ILLINOIS EYE AND EAR INFIRMARY: Treating the most serious and complicated ophthalmology cases for over 150 years
Acute Altitudinal Visual Field Defect with Optic Disc Edema: (Continued)<br />
FIGURE 1<br />
24-2 HVF done 2 weeks prior to presentation at UIC, demonstrates a dense superior altitudinal defect in the left eye.<br />
DISCUSSION The precise mechanism <strong>of</strong> or location <strong>of</strong> injury is unknown in NAION, or non-arteritic anterior ischemic optic neuropathy.<br />
Some have favored to calling the nonarteritic form <strong>of</strong> AION as idiopathic anterior ischemic optic neuropathy. It is presumed to be related to<br />
compromise <strong>of</strong> optic disc microcirculation in the setting <strong>of</strong> structural crowding <strong>of</strong> the disc. Ischemia, edema, and compartment syndrome<br />
due to optic disc structure are all thought to play a role. Signs and symptoms include sudden onset <strong>of</strong> painless vision loss and or associated<br />
visual field defect. A relative afferent pupillary defect is present in the affected eye and there is sectoral or generalized optic disc edema<br />
on examination. If the edema persists for over 8 weeks, an alternative diagnosis should be found. Optic disc filling is usually delayed on<br />
fluorescein angiography. Improvement in vision is reported in around 30% <strong>of</strong> patients after 2 years, according to the ischemic optic nerve<br />
decompression trial. NAION usually affects Caucasians over the age <strong>of</strong> 50, with no gender predilection. There is a 15% risk <strong>of</strong> contralateral<br />
involvement in 5 years. There is no proven treatment for NAION, however there are mixed reports about using aspirin for prophylaxis and<br />
levodopa as treatment in the literature.<br />
Primary VZV infection, which occurs in children, results in chicken pox, after which the virus becomes latent in ganglionic neurons. Years<br />
later, as cell mediated immunity to VZV declines with age or from immunosuppression, VZV can reactivate to cause zoster. Zoster is <strong>of</strong>ten<br />
followed by chronic pain as well as vasculopathy, myelopathy, retinal necrosis and cerebellitis. VZV reactivation can cause pain and all other<br />
neurological complications <strong>of</strong> VZV reactivation without rash. Optic nerve involvement may occur simultaneously with the acute vesicular<br />
rash or without a rash, or as a postherpetic complication.<br />
The earliest description <strong>of</strong> VZV vasculopathy was described as a noninfectious granulomatous angiitis with a predilection for the nervous<br />
system, characterized by thrombosis in cerebral arteries, consisting predominantly <strong>of</strong> histiocytes, mononuclear cells and multinucleated<br />
giant cells. Patients can present with stroke like symptoms, headaches, changes in mental status, hemianopia, or monocular vision loss.<br />
37% <strong>of</strong> cases <strong>of</strong> VZV vasculopathy develop without rash. Brain imaging reveals abnormalities – ischemic lesions usually at the grey-white<br />
matter junctions much like metastatic or embolic disease. Angiographic changes include segmental constriction with poststenotic diltation.<br />
Abnormalities in the CSF include modest pleocytosis with fewer than 100 cells, predominantely mononuclear. CSF protein is increased and<br />
glucose is normal. Oligoclonal bands are present. Anti VZV IgG antibodies are more sensitive than VZV DNA in the CSF for VZV vasculopathy;<br />
however both studies are encouraged for patients suspected <strong>of</strong> VZV vasculopathy. Treatment recommendations include IV acyclovir for<br />
10 to 14 days with or without concurrent corticosteroids. There is not enough evidence to prefer the use or the avoidance <strong>of</strong> corticosteroids<br />
in VZV vasculopathy.<br />
Back to our case, his MRI showed no additional pathology aside from small vessel ischemic changes seen previously and his lumbar<br />
puncture was within normal limits without evidence <strong>of</strong> VZV antibodies or DNA. Thus the diagnosis still is debatable between an idiopathic<br />
anterior ischemic optic neuropathy as opposed to ischemic optic neuropathy secondary to VZV vasculopathy.<br />
UIC Department <strong>of</strong> Ophthalmology and Visual Sciences, 1855 West Taylor Street, MC 648, Chicago, IL 60612
Acute Altitudinal Visual Field Defect with Optic Disc Edema: (Continued)<br />
REFERENCES:<br />
Arnold AC. Pathogenesis <strong>of</strong> noarteritic anterior ischemic optic neuropathy. J Neuro-Ophthalmol 2003; 23:157-163.<br />
Buono LM et al. Nonarteritic anterior ischemic optic neuropathy. Curr Opin Ophthalmol 2002, 13: 357-561.<br />
Borruat FX and Herbort, CP. Herpes zoster ophthalmicus: anterior ischemic optic neuropathy and acyclovir. J Clin Neuro-ophthalmology,<br />
1992; 12(1): 37-40.<br />
Gilden DH et al. Two patients with unusual forms <strong>of</strong> varicella-zoster virus vasculopathy. N Engl J Med; 2002: 347 (19): 1500-3.<br />
Gilden DH et al. Varicella zoster virus vasculopathies: diverse cliinical manifestations, laboratory features pathogenesis, and treatment. Lancet<br />
Neurol, 2009; 8:731-40.<br />
Salazar R et al. Varicella zoster virus ischemic optic neuropathy and subclinical temporal artery involvement. Arch Neurol, 2011; 68(4): 517-<br />
520.<br />
Wenkel H et al. Detection <strong>of</strong> varicella zoster virus DNA and viral antigen in human eyes after herpes zoster ophthalmicus. Ophthalmology,<br />
1998; 105: 1323-1330.<br />
Complicated Orbital Floor Repair: Mark Krakauer, <strong>MD</strong> (Resident)<br />
MF is a 41 year old man who was referred to the UIC ED by an outside plastic surgeon for acute vision loss. He had<br />
a history <strong>of</strong> right orbital fracture without globe injury from blunt trauma from a bottle in 1991, and underwent fracture<br />
repair in 1992. He had subsequent enophthalmos without diplopia and underwent repair <strong>of</strong> the fracture earlier that day<br />
by the plastic surgeon. 3 titanium plates were placed to repair the floor and medial wall fracture. After awakening from<br />
surgery, the patient reported decreased vision. Ophthalmology was consulted.<br />
CT orbits without contrast showed one medial wall plate curved over the medial rectus and effacing the nerve. The<br />
right optic nerve was smaller in diameter than the left. There was intraorbital edema and an irregular and distorted<br />
medial rectus (see Figures 1 and 2). The patient was taken back to the operating room for emergent repositioning <strong>of</strong><br />
the plates. A repeat CT showed no impingement on the nerve although the right nerve still appeared smaller than the<br />
left. The patient’s vision did not improve postoperatively. The patient was started on Solumedrol 250 mg IV every 6 hrs.<br />
The patient was sent to the UIC ED the next day.<br />
On exam in the UIC ED, the patient’s vision in the right eye was light perception with projection and 20/25 in the left eye. On Ishihara testing, he<br />
was 0/11 OD and 11/11 OS. Motility exam showed -3 abduction and -4 in all other directions OD and full OS. Pupils were 5mm, fixed and middilated<br />
with a large rAPD OD and 3 to 1 mm OS. Confrontation visual fields were unable OD and full OS. Hertel was 24 mm OD and 22 mm OS<br />
at base 124. He had normal facial sensation.<br />
External exam showed nearly complete ptosis and 0.5 mm lagophthalmos, 2+ periorbital edema and a subciliary right lower lid incision w/sutures.<br />
MRD1 was -4 OD and 5 OS. LF showed 0 OD and 18 OS. PF showed 0.5 OD and 9 OS. Applanation pressure was 20 OD and 18 OS. Anterior<br />
segment exam showed subconjunctival heme inferiorly. Posterior segement exam was normal; the nerve showed no edema or pallor.<br />
THE ILLINOIS EYE AND EAR INFIRMARY: Treating the most serious and complicated ophthalmology cases for over 150 years
Complicated Orbital Floor Repair: (Continued)<br />
FIGURE 1: CT scan orbits, sequential coronal cuts<br />
demonstrating impingement <strong>of</strong> orbital implants on<br />
right optic nerve.<br />
FIGURE 2: CT scan orbit, axial sequence showing<br />
orbital implant pressing upon the optic nerve.<br />
UIC Department <strong>of</strong> Ophthalmology and Visual Sciences, 1855 West Taylor Street, MC 648, Chicago, IL 60612
Complicated Orbital Floor Repair: (Continued)<br />
In summary, the patient had right optic neuropathy from compression and/or ischemia, with ophthalmoplegia from postoperative edema vs.<br />
CN3 palsy (lid and pupil involved). The patient was started on IV solumedrol 250 mg IV every 6 hrs for 3 days and cephalexin 500 mg PO QID<br />
for 7 days. On postoperative day 11, the vision decreased to no light perception with the remainder <strong>of</strong> the exam unchanged. At postoperative<br />
month three, the vision was still no light perception, although motility and lid function improved. There was temporal disc pallor. At postoperative<br />
month six, the exam was unchanged.<br />
DISCUSSION Direct traumatic optic neuropathy occurs from an anatomical disruption <strong>of</strong> the optic nerve, typically resulting in severe and<br />
immediate vision loss with a poor prognosis. Indirect traumatic optic neuropathy, as in this case, is due to transmission <strong>of</strong> forces to the<br />
optic nerve from a distant site without disruption <strong>of</strong> normal tissue structures (blunt trauma). Primary injury occurs from mechanical shearing<br />
<strong>of</strong> axons and vessels because the intracanalicular optic nerve dural sheath is adherent to periosteum. Secondary injury occurs from edema<br />
causing compression and ischemia.<br />
In a case series on intraorbital foreign body (Fulcher 2002), 4 patients had traumatic optic neuropathy; all had vision less than 6/60 at presentation<br />
and did not improve. One case report <strong>of</strong> a patient with thyroid eye disease who underwent bilateral endoscopic orbital decompression<br />
had vision loss to hand motion due to a fragment <strong>of</strong> lamina papyracea in contact with the optic nerve. The fragment was removed,<br />
the orbit further decompressed, and vision improved to 20/20 at postoperative month four (Nazir 2009).<br />
REFERENCES:<br />
Nazir SA, Westfall CT, Chacko JG, Phillips PH, Stack BC Jr. Visual recovery after direct traumatic optic neuropathy. Am J Otolaryngol. 2010<br />
May-Jun;31(3):193-4. Epub 2009 Mar 27.<br />
Fulcher TP, McNab AA, Sullivan TJ. Clinical features and management <strong>of</strong> intraorbital foreign bodies. Ophthalmology. 2002 Mar;109(3):494-<br />
500.<br />
UPCOMING CME COURSES/EVENTS<br />
August 1-4, 2012 Midwest Ocular Angiography<br />
Conference<br />
August 10-12, 2012 Advanced Vitreoretinal Techniques<br />
& Technology (AVTT)<br />
September 14, 2012 2012 Oculoplastics Symposium<br />
October 5, 2012 Cless Best <strong>of</strong> the Best <strong>of</strong> ARVO<br />
and AAO for 2011<br />
November 10, 2012 AAO UIC Alumni Reception-Chicago<br />
Upcoming<br />
Illinois Eye and Ear Infirmary Ophthalmology Grand Rounds<br />
are held Wednesdays at 5:00 pm on the UIC campus at 909<br />
S. Wolcott in the College <strong>of</strong> Medicine Research Building. For<br />
a complete schedule go to www.chicago.medicine.uic.edu.<br />
Click on Education then Grand Rounds.<br />
Or, call 312-996-6590.<br />
THE ILLINOIS EYE AND EAR INFIRMARY: Treating the most serious and complicated ophthalmology cases for over 150 years













![CV Joan [W51] - University of Illinois College of Medicine at Chicago ...](https://img.yumpu.com/17336863/1/190x245/cv-joan-w51-university-of-illinois-college-of-medicine-at-chicago-.jpg?quality=85)