Dehydration of Cyclohexanol – Preparation of an Alkene
Dehydration of Cyclohexanol – Preparation of an Alkene
Dehydration of Cyclohexanol – Preparation of an Alkene
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Dehydration</strong> <strong>of</strong> <strong>Cyclohex<strong>an</strong>ol</strong> <strong>–</strong> <strong>Preparation</strong> <strong>of</strong> <strong>an</strong> <strong>Alkene</strong> CHM 220<br />
INTRODUCTION<br />
Earlier this semester you reacted <strong>an</strong> alcohol (n-but<strong>an</strong>ol) with a Hydro-halide acid (Hydro-bromic Acid) to<br />
generate <strong>an</strong> alkyl halide. This reaction occurred through the SN2 pathway. In this experiment you will<br />
react <strong>an</strong> alcohol (cyclohex<strong>an</strong>ol) utilizing <strong>an</strong> acid catalyst (sulfuric acid) to “dehydrate” the alcohol <strong>an</strong>d<br />
form <strong>an</strong> alkene (cyclohexene).<br />
Alcohols are frequently converted into the desired alkene using <strong>an</strong> acid catalyzed Elimination reaction.<br />
The term “dehydrate” me<strong>an</strong>s to remove water <strong>an</strong>d is used to identify the nature <strong>of</strong> the atoms/molecules<br />
eliminated to form the double bond <strong>of</strong> the alkene in our product. Primary (1 O ) alcohols require strong<br />
acids <strong>an</strong>d signific<strong>an</strong>t amounts <strong>of</strong> heat (up to 180 O C or more). Secondary alcohols (2 O ) are somewhat<br />
easier to dehydrate requiring slightly lower temperatures, where Tertiary (3 O ) alcohols will undergo<br />
dehydration at room temperature or only slightly above that.<br />
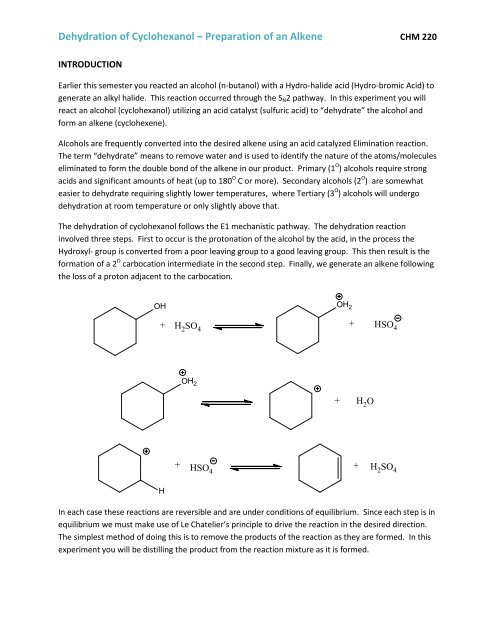
The dehydration <strong>of</strong> cyclohex<strong>an</strong>ol follows the E1 mech<strong>an</strong>istic pathway. The dehydration reaction<br />
involved three steps. First to occur is the protonation <strong>of</strong> the alcohol by the acid, in the process the<br />
Hydroxyl- group is converted from a poor leaving group to a good leaving group. This then result is the<br />
formation <strong>of</strong> a 2 O carbocation intermediate in the second step. Finally, we generate <strong>an</strong> alkene following<br />
the loss <strong>of</strong> a proton adjacent to the carbocation.<br />
OH<br />
OH 2<br />
+ H2SO + HSO<br />
4<br />
4<br />
H<br />
OH 2<br />
+ HSO4<br />
+ H 2 O<br />
+<br />
H 2 SO 4<br />
In each case these reactions are reversible <strong>an</strong>d are under conditions <strong>of</strong> equilibrium. Since each step is in<br />
equilibrium we must make use <strong>of</strong> Le Chatelier’s principle to drive the reaction in the desired direction.<br />
The simplest method <strong>of</strong> doing this is to remove the products <strong>of</strong> the reaction as they are formed. In this<br />
experiment you will be distilling the product from the reaction mixture as it is formed.
<strong>Dehydration</strong> <strong>of</strong> <strong>Cyclohex<strong>an</strong>ol</strong> <strong>–</strong> <strong>Preparation</strong> <strong>of</strong> <strong>an</strong> <strong>Alkene</strong> CHM 220<br />
PROCEDURE: (take care to record <strong>an</strong>y necessary data for your lab report)<br />
1) To a 50mL round bottom flask add 10mL <strong>of</strong> water <strong>an</strong>d then slowly add 10mL <strong>of</strong> concentrated<br />
sulfuric acid. Cool the mixture to room temperature, <strong>an</strong>d add a few boiling chips to the cooled<br />
solution.<br />
2) Prepare a distillation apparatus using a claisen adapter, three-way adapter, distillation<br />
condenser, thermometer adapter, thermometer(as shown below). Arr<strong>an</strong>ge the glassware on<br />
the wire grid in the hood so that you c<strong>an</strong> distill the product into a 25mL graduated cylinder.<br />
100 mL<br />
Water out<br />
Water in<br />
Ice Water<br />
3) Open the system, via the stopper, <strong>an</strong>d add 10 mL <strong>of</strong> cyclohex<strong>an</strong>ol using a funnel. Close the<br />
system <strong>an</strong>d begin heating with a heating m<strong>an</strong>tle. Heat the solution until it begins to boil <strong>an</strong>d<br />
then proceed to distill approximately 10mL <strong>of</strong> the crude cyclohexene product into the graduated<br />
cylinder.<br />
4) In a separatory funnel, wash the crude cyclohexene with two 10mL portions <strong>of</strong> a 10% sodium<br />
bicarbonate solution to remove <strong>an</strong>y excess acid. Remember to vent the funnel <strong>of</strong>ten. Discard<br />
the aqueous washes.<br />
5) Wash the remaining org<strong>an</strong>ic layer (cyclohexene) with one portion <strong>of</strong> saturated salt solution, <strong>an</strong>d<br />
then one portion <strong>of</strong> R.O. water. Again discarding the aqueous layers.<br />
6) Tr<strong>an</strong>sfer the cyclohexene to a beaker or flask <strong>an</strong>d add a small amount <strong>of</strong> calcium chloride to dry<br />
(remove <strong>an</strong>y excess water) the solution.<br />
7) If the product is NOT clear, centrifuge to complete the drying process.<br />
8) Dec<strong>an</strong>t the clear product to a tared weighing bottle to determine your yield (<strong>an</strong>d percent yield).
<strong>Dehydration</strong> <strong>of</strong> <strong>Cyclohex<strong>an</strong>ol</strong> <strong>–</strong> <strong>Preparation</strong> <strong>of</strong> <strong>an</strong> <strong>Alkene</strong> CHM 220<br />
Procedure: continued<br />
9) Determine the refractive index <strong>of</strong> your product.<br />
10) Take a small amount <strong>of</strong> your product <strong>an</strong>d fill a sample vial <strong>an</strong>d speak with your instructor on<br />
how to determine the purity <strong>of</strong> your product via Gas Chromatography (see G.C. Introduction on<br />
the CHM 220 lab website).<br />
11) Obtain <strong>an</strong> IR spectrum <strong>of</strong> your product.<br />
12) Test the remaining amount <strong>of</strong> your product using Br2 in hex<strong>an</strong>e, <strong>an</strong>d record your observations<br />
(refer to the Addition lab for more information).<br />
Questions to consider in your lab report:<br />
1) When a straight chain 2 O alcohol is dehydrated you will <strong>of</strong>ten obtain a mixture <strong>of</strong> products, see<br />
example below. Why do we only obtain a single product from cyclohex<strong>an</strong>ol instead <strong>of</strong> a<br />
mixture?<br />
OH<br />
H 2 SO 4<br />
heat<br />
ma j or<br />
minor<br />
+<br />
minor<br />
2) What would happen if you allowed the cyclohexene to st<strong>an</strong>d in contact with <strong>an</strong> aqueous sulfuric<br />
acid solution?<br />
3) Which compound has a higher boiling point, cyclohex<strong>an</strong>ol or cyclohexene?