marc lémann,* jean–yves mary,‡ bernard duclos,§ michel - IG-IBD
marc lémann,* jean–yves mary,‡ bernard duclos,§ michel - IG-IBD
marc lémann,* jean–yves mary,‡ bernard duclos,§ michel - IG-IBD
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
1058 LEMANN ET AL GASTROENTEROLOGY Vol. 130, No. 4<br />
Table 1. Baseline Characteristics of the Patients Enrolled in the Trial According to Study Treatment and Stratum<br />
was not different in the 2 treatment groups at 2.0 (.0–4.0)<br />
vs 2.0 (1.0–4.0) at week 6 (P .65), 2.0 (.0–3.0) vs 1.0<br />
(.0–3.0) at week 12 (P .91), and 1.0 (.0–2.0) vs .5<br />
(.0–1.8) at week 24 (P .42). There was no significant<br />
interaction between treatment and stratum.<br />
Factors associated with success at week 24 in the<br />
multivariate logistic regression analysis were a low CDAI<br />
at baseline (OR, 4.5; 95% CI, 1.5–13.7; P .005), a<br />
Failure stratum Naive stratum<br />
Placebo n 29 Infliximab n 26 P Placebo n 27 Infliximab n 31 P<br />
Sex (female) a 69 69 .95 43 39 .75<br />
Age, yb 29 (23–33) 26 (22–37) .52 26 (22–36) 27 (22–38) .79<br />
Disease duration, yb Disease location<br />
7 (3–11) 5 (4–10) .97 4 (1–8) 3 (1–6) .60<br />
a<br />
Small bowel 11 20 .37 15 35 .74<br />
Colon 50 32 26 13<br />
Both 39 48 59 52<br />
Active perianal diseasea 14 35 .07 7 32 .02<br />
CDAIb 181 (154–259) 240 (219–281) .02 112 (42–262) 146 (90–244) .48<br />
Duration of steroids, mob 16 (9–36) 25 (13–36) .20 15 (9–52) 9 (7–22) .03<br />
Steroid dose, mg/dayb 30 (20–40) 33 (20–46) .32 25 (20–40) 25 (20–40) .85<br />
Steroid dose, mg/kg/dayb .5 (.3–.7) .6 (.4–.8) .25 .4 (.3–.6) .4 (.3–.6) .77<br />
Duration of AZA/6-MP, mob 21 (10–44) 27 (10–33) .95 0 0 -<br />
Dosage of AZA, c mg/kg/dayb 2.4 (2.1–2.6) 2.3 (2.0–2.5) .27 2.2 (2.0–2.4) 2.2 (2.0–2.4) .94<br />
Dosage of AZA, c mg/dayb 150 (125–150) 125 (100–150) .39 125 (100–150) 150 (125–150) .20<br />
CDEISb (n 52) 9 (6–15) d 9 (4–14) e .63 6 (3–14) f 11 (5–16) g .50<br />
C-reactive protein, b mg/L 8 (4–35) 19 (4–47) h .63 17 (7–31) i 20 (10–30) .36<br />
a Percent of patients; 2 test.<br />
b Median (interquartile range); Mann–Whitney U test.<br />
c All patients were treated with AZA.<br />
d n 17.<br />
e n 9.<br />
f n 14.<br />
g n 12.<br />
h n 25.<br />
i n 26.<br />
young age (OR, 5.9; 95% CI, 1.8–19.6; P .002), the<br />
absence of small-bowel involvement (OR, 4.3; 95% CI,<br />
1.5–12.2; P .004), and a long duration of steroids<br />
before entry (OR, 5.5; 95% CI, 1.3–22.5; P .01).<br />
After adjusting for these factors, infliximab still was<br />
superior to placebo (OR, 5.7; 95% CI, 2.0–15.9; P<br />
.001) and no interaction between treatment and strata<br />
could be seen (P .63).<br />
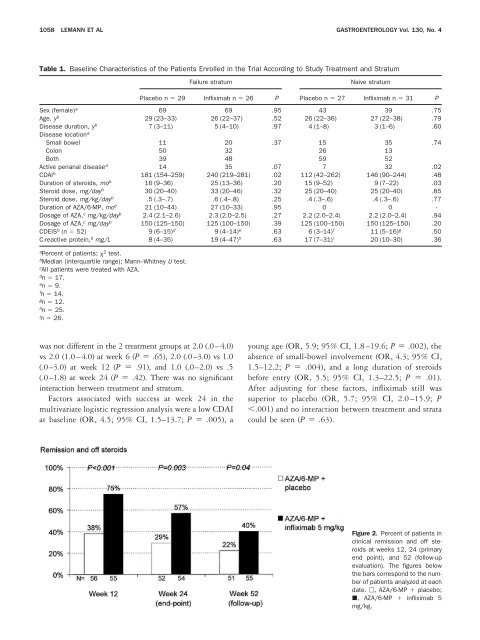
Figure 2. Percent of patients in<br />
clinical remission and off steroids<br />
at weeks 12, 24 (pri<strong>mary</strong><br />
end point), and 52 (follow-up<br />
evaluation). The figures below<br />
the bars correspond to the number<br />
of patients analyzed at each<br />
date. □, AZA/6-MP placebo;<br />
, AZA/6-MP infliximab 5<br />
mg/kg.