marc lémann,* jean–yves mary,‡ bernard duclos,§ michel - IG-IBD
marc lémann,* jean–yves mary,‡ bernard duclos,§ michel - IG-IBD
marc lémann,* jean–yves mary,‡ bernard duclos,§ michel - IG-IBD
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
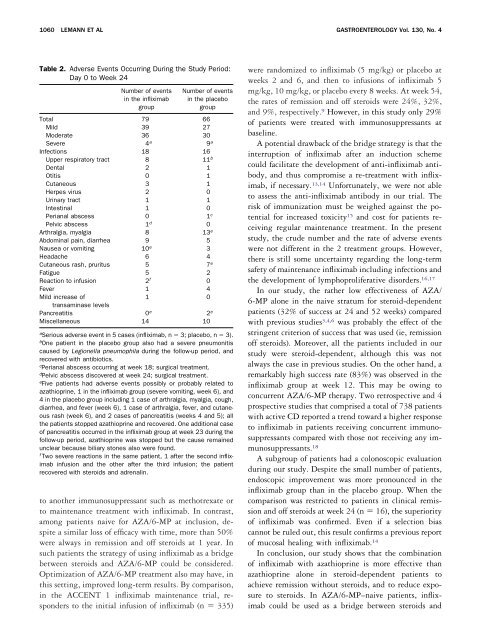
1060 LEMANN ET AL GASTROENTEROLOGY Vol. 130, No. 4<br />
Table 2. Adverse Events Occurring During the Study Period:<br />
Day 0 to Week 24<br />
Number of events<br />
in the infliximab<br />
group<br />
Number of events<br />
in the placebo<br />
group<br />
Total 79 66<br />
Mild 39 27<br />
Moderate 36 30<br />
Severe 4 a 9 a<br />
Infections 18 16<br />
Upper respiratory tract 8 11 b<br />
Dental 2 1<br />
Otitis 0 1<br />
Cutaneous 3 1<br />
Herpes virus 2 0<br />
Urinary tract 1 1<br />
Intestinal 1 0<br />
Perianal abscess 0 1 c<br />
Pelvic abscess 1 d 0<br />
Arthralgia, myalgia 8 13 e<br />
Abdominal pain, diarrhea 9 5<br />
Nausea or vomiting 10 e 3<br />
Headache 6 4<br />
Cutaneous rash, pruritus 5 7 e<br />
Fatigue 5 2<br />
Reaction to infusion 2 f 0<br />
Fever 1 4<br />
Mild increase of<br />
transaminase levels<br />
1 0<br />
Pancreatitis 0 e 2 e<br />
Miscellaneous 14 10<br />
a Serious adverse event in 5 cases (infliximab, n 3; placebo, n 3).<br />
b One patient in the placebo group also had a severe pneumonitis<br />
caused by Legionella pneumophila during the follow-up period, and<br />
recovered with antibiotics.<br />
c Perianal abscess occurring at week 18; surgical treatment.<br />
d Pelvic abscess discovered at week 24; surgical treatment.<br />
e Five patients had adverse events possibly or probably related to<br />
azathioprine, 1 in the infliximab group (severe vomiting, week 6), and<br />
4 in the placebo group including 1 case of arthralgia, myalgia, cough,<br />
diarrhea, and fever (week 6), 1 case of arthralgia, fever, and cutaneous<br />
rash (week 6), and 2 cases of pancreatitis (weeks 4 and 5); all<br />
the patients stopped azathioprine and recovered. One additional case<br />
of pancreatitis occurred in the infliximab group at week 23 during the<br />
follow-up period, azathioprine was stopped but the cause remained<br />
unclear because biliary stones also were found.<br />
f Two severe reactions in the same patient, 1 after the second infliximab<br />
infusion and the other after the third infusion; the patient<br />
recovered with steroids and adrenalin.<br />
to another immunosuppressant such as methotrexate or<br />
to maintenance treatment with infliximab. In contrast,<br />
among patients naive for AZA/6-MP at inclusion, despite<br />
a similar loss of efficacy with time, more than 50%<br />
were always in remission and off steroids at 1 year. In<br />
such patients the strategy of using infliximab as a bridge<br />
between steroids and AZA/6-MP could be considered.<br />
Optimization of AZA/6-MP treatment also may have, in<br />
this setting, improved long-term results. By comparison,<br />
in the ACCENT 1 infliximab maintenance trial, responders<br />
to the initial infusion of infliximab (n 335)<br />
were randomized to infliximab (5 mg/kg) or placebo at<br />
weeks 2 and 6, and then to infusions of infliximab 5<br />
mg/kg, 10 mg/kg, or placebo every 8 weeks. At week 54,<br />
the rates of remission and off steroids were 24%, 32%,<br />
and 9%, respectively. 9 However, in this study only 29%<br />
of patients were treated with immunosuppressants at<br />
baseline.<br />
A potential drawback of the bridge strategy is that the<br />
interruption of infliximab after an induction scheme<br />
could facilitate the development of anti-infliximab antibody,<br />
and thus compromise a re-treatment with infliximab,<br />
if necessary. 13,14 Unfortunately, we were not able<br />
to assess the anti-infliximab antibody in our trial. The<br />
risk of immunization must be weighed against the potential<br />
for increased toxicity 15 and cost for patients receiving<br />
regular maintenance treatment. In the present<br />
study, the crude number and the rate of adverse events<br />
were not different in the 2 treatment groups. However,<br />
there is still some uncertainty regarding the long-term<br />
safety of maintenance infliximab including infections and<br />
the development of lymphoproliferative disorders. 16,17<br />
In our study, the rather low effectiveness of AZA/<br />
6-MP alone in the naive stratum for steroid-dependent<br />
patients (32% of success at 24 and 52 weeks) compared<br />
with previous studies 3,4,6 was probably the effect of the<br />
stringent criterion of success that was used (ie, remission<br />
off steroids). Moreover, all the patients included in our<br />
study were steroid-dependent, although this was not<br />
always the case in previous studies. On the other hand, a<br />
remarkably high success rate (83%) was observed in the<br />
infliximab group at week 12. This may be owing to<br />
concurrent AZA/6-MP therapy. Two retrospective and 4<br />
prospective studies that comprised a total of 738 patients<br />
with active CD reported a trend toward a higher response<br />
to infliximab in patients receiving concurrent immunosuppressants<br />
compared with those not receiving any immunosuppressants.<br />
18<br />
A subgroup of patients had a colonoscopic evaluation<br />
during our study. Despite the small number of patients,<br />
endoscopic improvement was more pronounced in the<br />
infliximab group than in the placebo group. When the<br />
comparison was restricted to patients in clinical remission<br />
and off steroids at week 24 (n 16), the superiority<br />
of infliximab was confirmed. Even if a selection bias<br />
cannot be ruled out, this result confirms a previous report<br />
of mucosal healing with infliximab. 14<br />
In conclusion, our study shows that the combination<br />
of infliximab with azathioprine is more effective than<br />
azathioprine alone in steroid-dependent patients to<br />
achieve remission without steroids, and to reduce exposure<br />
to steroids. In AZA/6-MP–naive patients, infliximab<br />
could be used as a bridge between steroids and