Comparative Efficacy and Safety of Lisdexamfetamine Dimesylate ...
Comparative Efficacy and Safety of Lisdexamfetamine Dimesylate ...
Comparative Efficacy and Safety of Lisdexamfetamine Dimesylate ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
significantly lower in both MAS XR groups compared with<br />
3.4 (1.00) in the placebo group (P≤.0027; Table 2). Effect sizes<br />
for treatment with LDX <strong>and</strong> MAS XR as assessed by the global<br />
improvement scales are presented in Table 2.<br />
In the LDX trial, a post hoc analysis <strong>of</strong> dichotomized CGI-I<br />
difference in improved (very much improved [CGI-I=1] <strong>and</strong><br />
much improved [CGI-I=2]) versus placebo was significant for<br />
both doses <strong>of</strong> LDX, at all weeks <strong>and</strong> at endpoint (P≤.0005 for<br />
each). The percentage <strong>of</strong> subjects for 50 <strong>and</strong> 70 mg/day LDX,<br />
respectively, at week 1 was 24.7% <strong>and</strong> 27.9%; at week 2 was<br />
32.5% <strong>and</strong> 34.2%; at week 3 was 35.1% <strong>and</strong> 38.9%; at week 4<br />
was 32.9% <strong>and</strong> 33.3%; <strong>and</strong> at endpoint was 32.5% <strong>and</strong> 31.8%.<br />
For the MAS XR trial, the analysis indicated that the categorical<br />
CGI-I difference in improved versus placebo was not significant<br />
for the 20 mg/day dose <strong>of</strong> MAS XR at all weeks <strong>and</strong> at endpoint.<br />
With the 40 mg/day dose <strong>of</strong> MAS XR, the percentage <strong>of</strong> subjects<br />
that had a difference in improved versus placebo was significant<br />
only at weeks 2 <strong>and</strong> 4, <strong>and</strong> at endpoint (P≤.0210 for each). The<br />
CGI-I difference for the 40 mg/day dose <strong>of</strong> MAS XR at week 2<br />
was 27.3%; at week 4 was 29.2%; <strong>and</strong> at endpoint was 29.1%.<br />
<strong>Safety</strong><br />
The harmonization <strong>of</strong> AE terminology resulted in the reclassification<br />
<strong>of</strong> the verbatim item mapping to the COSTART<br />
“anorexia” to the MedDRA “decreased appetite” or “anorexia.”<br />
The verbatim terms mapping to the COSTART “insomnia” was<br />
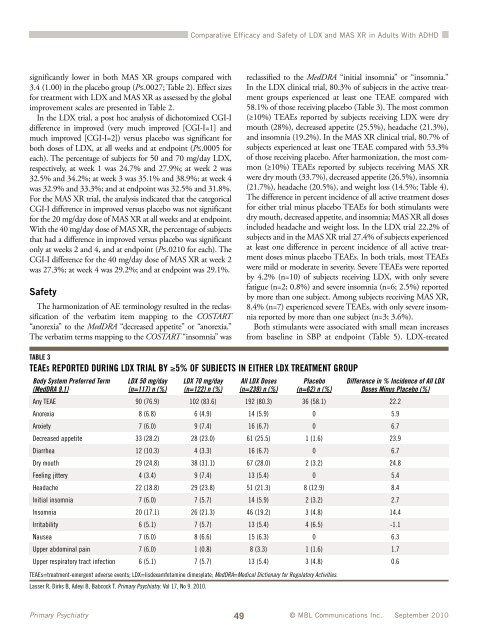
TABLE 3<br />
<strong>Comparative</strong> <strong>Efficacy</strong> <strong>and</strong> <strong>Safety</strong> <strong>of</strong> LDX <strong>and</strong> MAS XR in Adults With ADHD<br />
reclassified to the MedDRA “initial insomnia” or “insomnia.”<br />
In the LDX clinical trial, 80.3% <strong>of</strong> subjects in the active treatment<br />
groups experienced at least one TEAE compared with<br />
58.1% <strong>of</strong> those receiving placebo (Table 3). The most common<br />
(≥10%) TEAEs reported by subjects receiving LDX were dry<br />
mouth (28%), decreased appetite (25.5%), headache (21.3%),<br />
<strong>and</strong> insomnia (19.2%). In the MAS XR clinical trial, 80.7% <strong>of</strong><br />
subjects experienced at least one TEAE compared with 53.3%<br />
<strong>of</strong> those receiving placebo. After harmonization, the most common<br />
(≥10%) TEAEs reported by subjects receiving MAS XR<br />
were dry mouth (33.7%), decreased appetite (26.5%), insomnia<br />
(21.7%), headache (20.5%), <strong>and</strong> weight loss (14.5%; Table 4).<br />
The difference in percent incidence <strong>of</strong> all active treatment doses<br />
for either trial minus placebo TEAEs for both stimulants were<br />
dry mouth, decreased appetite, <strong>and</strong> insomnia; MAS XR all doses<br />
included headache <strong>and</strong> weight loss. In the LDX trial 22.2% <strong>of</strong><br />
subjects <strong>and</strong> in the MAS XR trial 27.4% <strong>of</strong> subjects experienced<br />
at least one difference in percent incidence <strong>of</strong> all active treatment<br />
doses minus placebo TEAEs. In both trials, most TEAEs<br />
were mild or moderate in severity. Severe TEAEs were reported<br />
by 4.2% (n=10) <strong>of</strong> subjects receiving LDX, with only severe<br />
fatigue (n=2; 0.8%) <strong>and</strong> severe insomnia (n=6; 2.5%) reported<br />
by more than one subject. Among subjects receiving MAS XR,<br />
8.4% (n=7) experienced severe TEAEs, with only severe insomnia<br />
reported by more than one subject (n=3; 3.6%).<br />
Both stimulants were associated with small mean increases<br />
from baseline in SBP at endpoint (Table 5). LDX-treated<br />
TEAES REPORTED DURING LDX TRIAL BY ≥5% OF SUBJECTS IN EITHER LDX TREATMENT GROUP<br />
Body System Preferred Term<br />
(MedDRA 9.1)<br />
LDX 50 mg/day<br />
(n=117) n (%)<br />
LDX 70 mg/day<br />
(n=122) n (%)<br />
All LDX Doses<br />
(n=239) n (%)<br />
Placebo<br />
(n=62) n (%)<br />
Difference in % Incidence <strong>of</strong> All LDX<br />
Doses Minus Placebo (%)<br />
Any TEAE 90 (76.9) 102 (83.6) 192 (80.3) 36 (58.1) 22.2<br />
Anorexia 8 (6.8) 6 (4.9) 14 (5.9) 0 5.9<br />
Anxiety 7 (6.0) 9 (7.4) 16 (6.7) 0 6.7<br />
Decreased appetite 33 (28.2) 28 (23.0) 61 (25.5) 1 (1.6) 23.9<br />
Diarrhea 12 (10.3) 4 (3.3) 16 (6.7) 0 6.7<br />
Dry mouth 29 (24.8) 38 (31.1) 67 (28.0) 2 (3.2) 24.8<br />
Feeling jittery 4 (3.4) 9 (7.4) 13 (5.4) 0 5.4<br />
Headache 22 (18.8) 29 (23.8) 51 (21.3) 8 (12.9) 8.4<br />
Initial insomnia 7 (6.0) 7 (5.7) 14 (5.9) 2 (3.2) 2.7<br />
Insomnia 20 (17.1) 26 (21.3) 46 (19.2) 3 (4.8) 14.4<br />
Irritability 6 (5.1) 7 (5.7) 13 (5.4) 4 (6.5) -1.1<br />
Nausea 7 (6.0) 8 (6.6) 15 (6.3) 0 6.3<br />
Upper abdominal pain 7 (6.0) 1 (0.8) 8 (3.3) 1 (1.6) 1.7<br />
Upper respiratory tract infection 6 (5.1) 7 (5.7) 13 (5.4) 3 (4.8) 0.6<br />
TEAEs=treatment-emergent adverse events; LDX=lisdexamfetamine dimesylate; MedDRA=Medical Dictionary for Regulatory Activities.<br />
Lasser R, Dirks B, Adeyi B, Babcock T. Primary Psychiatry. Vol 17, No 9. 2010.<br />
Primary Psychiatry 49<br />
© MBL Communications Inc. September 2010