Comparative Efficacy and Safety of Lisdexamfetamine Dimesylate ...
Comparative Efficacy and Safety of Lisdexamfetamine Dimesylate ...
Comparative Efficacy and Safety of Lisdexamfetamine Dimesylate ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
DISCUSSION<br />
This study is the first to qualitatively assess two long-acting,<br />
amphetamine-based stimulants, LDX <strong>and</strong> MAS XR, in<br />
adults. Using groups derived from registration trials that were<br />
matched on baseline severity <strong>of</strong> ADHD symptoms, duration<br />
<strong>of</strong> treatment, <strong>and</strong> approximately comparable amphetamine<br />
doses, a post hoc matched-group assessment was conducted.<br />
In these clinical trials, LDX <strong>and</strong> MAS XR had similar TEAEs.<br />
Common TEAEs ≥10% in the present analysis <strong>of</strong> both studies<br />
included dry mouth, decreased appetite, insomnia, <strong>and</strong><br />
headache. The common differences <strong>of</strong> the percent incidence<br />
<strong>of</strong> all active treatment doses minus placebo TEAEs in both trials<br />
were decreased appetite, insomnia, <strong>and</strong> dry mouth. These<br />
are consistent with TEAEs observed with other long-acting<br />
stimulants in adults. 39,40 Although most TEAEs in both clini-<br />
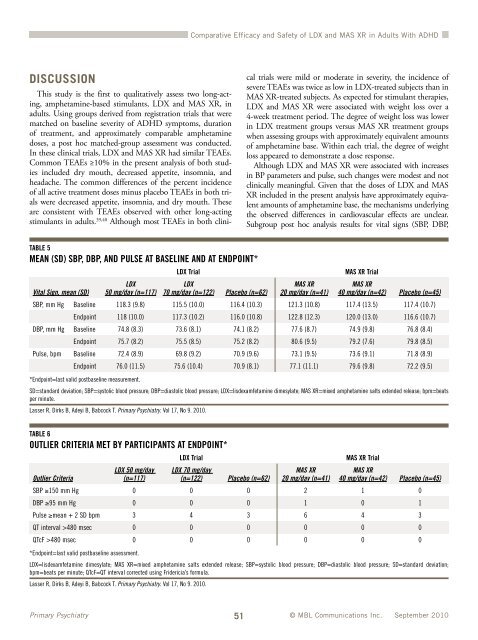
TABLE 5<br />
MEAN (SD) SBP, DBP, AND PULSE AT BASELINE AND AT ENDPOINT*<br />
Vital Sign, mean (SD)<br />
LDX<br />
50 mg/day (n=117)<br />
<strong>Comparative</strong> <strong>Efficacy</strong> <strong>and</strong> <strong>Safety</strong> <strong>of</strong> LDX <strong>and</strong> MAS XR in Adults With ADHD<br />
cal trials were mild or moderate in severity, the incidence <strong>of</strong><br />
severe TEAEs was twice as low in LDX-treated subjects than in<br />
MAS XR-treated subjects. As expected for stimulant therapies,<br />
LDX <strong>and</strong> MAS XR were associated with weight loss over a<br />
4-week treatment period. The degree <strong>of</strong> weight loss was lower<br />
in LDX treatment groups versus MAS XR treatment groups<br />
when assessing groups with approximately equivalent amounts<br />
<strong>of</strong> amphetamine base. Within each trial, the degree <strong>of</strong> weight<br />
loss appeared to demonstrate a dose response.<br />
Although LDX <strong>and</strong> MAS XR were associated with increases<br />
in BP parameters <strong>and</strong> pulse, such changes were modest <strong>and</strong> not<br />
clinically meaningful. Given that the doses <strong>of</strong> LDX <strong>and</strong> MAS<br />
XR included in the present analysis have approximately equivalent<br />
amounts <strong>of</strong> amphetamine base, the mechanisms underlying<br />
the observed differences in cardiovascular effects are unclear.<br />
Subgroup post hoc analysis results for vital signs (SBP, DBP,<br />
LDX Trial MAS XR Trial<br />
LDX<br />
70 mg/day (n=122) Placebo (n=62)<br />
MAS XR<br />
20 mg/day (n=41)<br />
MAS XR<br />
40 mg/day (n=42) Placebo (n=45)<br />
SBP, mm Hg Baseline 118.3 (9.8) 115.5 (10.0) 116.4 (10.3) 121.3 (10.8) 117.4 (13.5) 117.4 (10.7)<br />
Endpoint 118 (10.0) 117.3 (10.2) 116.0 (10.8) 122.8 (12.3) 120.0 (13.0) 116.6 (10.7)<br />
DBP, mm Hg Baseline 74.8 (8.3) 73.6 (8.1) 74.1 (8.2) 77.6 (8.7) 74.9 (9.8) 76.8 (8.4)<br />
Endpoint 75.7 (8.2) 75.5 (8.5) 75.2 (8.2) 80.6 (9.5) 79.2 (7.6) 79.8 (8.5)<br />
Pulse, bpm Baseline 72.4 (8.9) 69.8 (9.2) 70.9 (9.6) 73.1 (9.5) 73.6 (9.1) 71.8 (8.9)<br />
Endpoint 76.0 (11.5) 75.6 (10.4) 70.9 (8.1) 77.1 (11.1) 79.6 (9.8) 72.2 (9.5)<br />
*Endpoint=last valid postbaseline measurement.<br />
SD=st<strong>and</strong>ard deviation; SBP=systolic blood pressure; DBP=diastolic blood pressure; LDX=lisdexamfetamine dimesylate; MAS XR=mixed amphetamine salts extended release; bpm=beats<br />
per minute.<br />
Lasser R, Dirks B, Adeyi B, Babcock T. Primary Psychiatry. Vol 17, No 9. 2010.<br />
TABLE 6<br />
OUTLIER CRITERIA MET BY PARTICIPANTS AT ENDPOINT*<br />
Outlier Criteria<br />
LDX 50 mg/day<br />
(n=117)<br />
LDX Trial MAS XR Trial<br />
LDX 70 mg/day<br />
(n=122) Placebo (n=62)<br />
MAS XR<br />
20 mg/day (n=41)<br />
MAS XR<br />
40 mg/day (n=42) Placebo (n=45)<br />
SBP ≥150 mm Hg 0 0 0 2 1 0<br />
DBP ≥95 mm Hg 0 0 0 1 0 1<br />
Pulse ≥mean + 2 SD bpm 3 4 3 6 4 3<br />
QT interval >480 msec 0 0 0 0 0 0<br />
QTcF >480 msec 0 0 0 0 0 0<br />
*Endpoint=last valid postbaseline assessment.<br />
LDX=lisdexamfetamine dimesylate; MAS XR=mixed amphetamine salts extended release; SBP=systolic blood pressure; DBP=diastolic blood pressure; SD=st<strong>and</strong>ard deviation;<br />
bpm=beats per minute; QTcF=QT interval corrected using Fridericia’s formula.<br />
Lasser R, Dirks B, Adeyi B, Babcock T. Primary Psychiatry. Vol 17, No 9. 2010.<br />
Primary Psychiatry 51<br />
© MBL Communications Inc. September 2010