Experimental and numerical detonation cell in H2-N2O-Ar mixtures
Experimental and numerical detonation cell in H2-N2O-Ar mixtures
Experimental and numerical detonation cell in H2-N2O-Ar mixtures
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
ecomes effective <strong>in</strong>duc<strong>in</strong>g a large jump <strong>in</strong> the<br />
measured velocity. F<strong>in</strong>ally, as the (P4/P1) ratio<br />
cont<strong>in</strong>ues to <strong>in</strong>crease, the wave velocity tends to<br />
stabilize at the self-susta<strong>in</strong>ed CJ velocity. It can be<br />
noted that the critical pressure ratio is significantly<br />
reduced at higher <strong>in</strong>itial pressure ((P4/P1)≈35 for<br />
P1 = 20 kPa) due to the decrease of the critical<br />
energy for <strong>detonation</strong> onset. Critical conditions<br />
for the <strong>detonation</strong> onset have been estimated at<br />
several <strong>in</strong>itial pressures from the ideal 1-D shock<br />
wave theory, us<strong>in</strong>g the velocity measurements as a<br />
function of the (P4/P1) ratio. For P1 =10kPa,the<br />
critical temperature <strong>and</strong> pressure are 904 K <strong>and</strong> 127<br />
kPa, respectively. For P1 = 20 kPa, the critical temperature<br />
is 750 K <strong>and</strong> the critical pressure is 188 kPa.<br />
Wave velocity (m/s)<br />
2400<br />
2000<br />
1600<br />
1200<br />
800<br />
400<br />
<strong>Experimental</strong> data<br />
CJ velocity<br />
20 40 60 80<br />
P 4/P 1<br />
Figure 2: Wave velocity <strong>in</strong> <strong>H2</strong>-<strong>N2O</strong>-<strong>Ar</strong> mixture as a function<br />
of the (P4/P1) ratio. Initial conditions: Φ = 1; X<strong>Ar</strong><br />
=0.5;P1 =10kPa;T1 = 295 K<br />
λ (mm)<br />
10<br />
80<br />
70<br />
60<br />
50<br />
40<br />
30<br />
20<br />
X <strong>Ar</strong> = 0.2<br />
X <strong>Ar</strong> = 0.4<br />
X <strong>Ar</strong> = 0.6<br />
1<br />
Equivalence ratio<br />
Figure 3: Detonation <strong>cell</strong> size <strong>in</strong> <strong>H2</strong>-<strong>N2O</strong>-<strong>Ar</strong> <strong>mixtures</strong><br />
as a function of equivalence ratio at different dilutions.<br />
Initial conditions: Φ = 0.3-2.5; X<strong>Ar</strong> = 0.2-0.6; P1 =10<br />
kPa; T1 = 295 K<br />
3<br />
λ (mm)<br />
100<br />
10<br />
1<br />
P 1 = 7 kPa<br />
P 1 = 10 kPa<br />
P 1 = 35 kPa<br />
1<br />
Equivalence ratio<br />
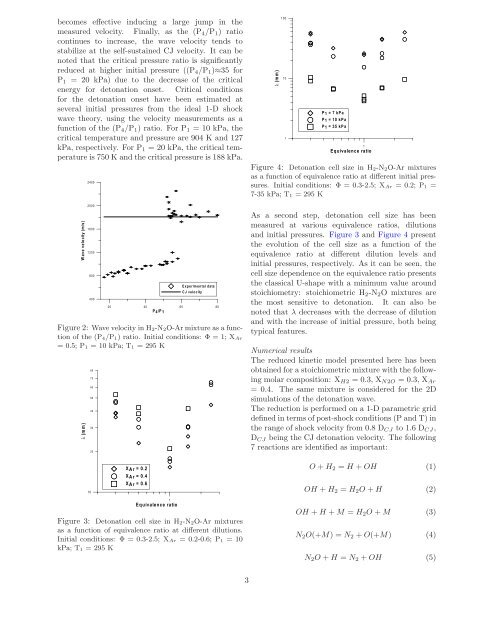
Figure 4: Detonation <strong>cell</strong> size <strong>in</strong> <strong>H2</strong>-<strong>N2O</strong>-<strong>Ar</strong> <strong>mixtures</strong><br />
as a function of equivalence ratio at different <strong>in</strong>itial pressures.<br />
Initial conditions: Φ = 0.3-2.5; X<strong>Ar</strong> =0.2;P1 =<br />
7-35 kPa; T1 = 295 K<br />
As a second step, <strong>detonation</strong> <strong>cell</strong> size has been<br />
measured at various equivalence ratios, dilutions<br />
<strong>and</strong> <strong>in</strong>itial pressures. Figure 3 <strong>and</strong> Figure 4 present<br />
the evolution of the <strong>cell</strong> size as a function of the<br />
equivalence ratio at different dilution levels <strong>and</strong><br />
<strong>in</strong>itial pressures, respectively. As it can be seen, the<br />
<strong>cell</strong> size dependence on the equivalence ratio presents<br />
the classical U-shape with a m<strong>in</strong>imum value around<br />
stoichiometry: stoichiometric <strong>H2</strong>-<strong>N2O</strong> <strong>mixtures</strong> are<br />
the most sensitive to <strong>detonation</strong>. It can also be<br />
noted that λ decreases with the decrease of dilution<br />
<strong>and</strong> with the <strong>in</strong>crease of <strong>in</strong>itial pressure, both be<strong>in</strong>g<br />
typical features.<br />
Numerical results<br />
The reduced k<strong>in</strong>etic model presented here has been<br />
obta<strong>in</strong>ed for a stoichiometric mixture with the follow<strong>in</strong>g<br />
molar composition: X<strong>H2</strong> =0.3,X<strong>N2O</strong> =0.3,X<strong>Ar</strong><br />
= 0.4. The same mixture is considered for the 2D<br />
simulations of the <strong>detonation</strong> wave.<br />
The reduction is performed on a 1-D parametric grid<br />
def<strong>in</strong>ed <strong>in</strong> terms of post-shock conditions (P <strong>and</strong> T) <strong>in</strong><br />
the range of shock velocity from 0.8 DCJ to 1.6 DCJ,<br />
DCJ be<strong>in</strong>g the CJ <strong>detonation</strong> velocity. The follow<strong>in</strong>g<br />
7 reactions are identified as important:<br />
O + <strong>H2</strong> = H + OH (1)<br />
OH + <strong>H2</strong> = <strong>H2</strong>O + H (2)<br />
OH + H + M = <strong>H2</strong>O + M (3)<br />
<strong>N2O</strong>(+M) =N2 + O(+M) (4)<br />
<strong>N2O</strong> + H = N2 + OH (5)