Experimental and numerical detonation cell in H2-N2O-Ar mixtures
Experimental and numerical detonation cell in H2-N2O-Ar mixtures
Experimental and numerical detonation cell in H2-N2O-Ar mixtures
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
a function of the equivalence ratio, <strong>in</strong>itial pressure<br />
<strong>and</strong> dilution is well reproduced by the correlation.<br />
As important parameters of the correlation (the<br />
<strong>in</strong>duction distance <strong>and</strong> the reaction zone length)<br />
are derived from the detailed scheme, it can be<br />
concluded that the accuracy of the predicted <strong>cell</strong><br />
size is directly related to the quality of the k<strong>in</strong>etic<br />
scheme. Thus, the correlation of Ng can be used as a<br />
reliable tool as long as a carefully validated detailed<br />
chemical model is used.<br />
Further, 2-D <strong>numerical</strong> simulations have been performed.<br />
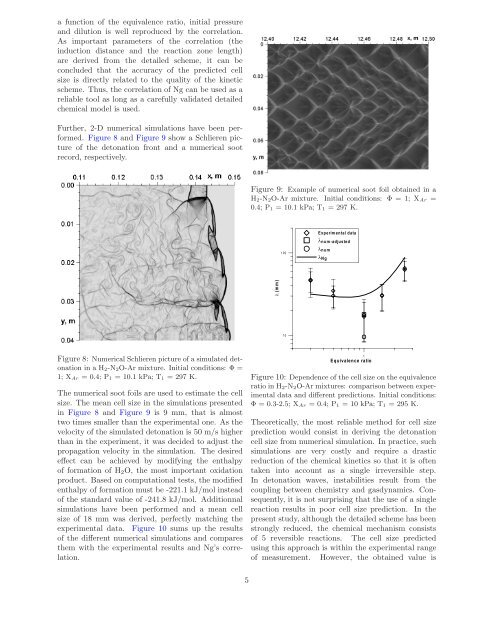
Figure 8 <strong>and</strong> Figure 9 show a Schlieren picture<br />
of the <strong>detonation</strong> front <strong>and</strong> a <strong>numerical</strong> soot<br />
record, respectively.<br />
Figure 8: Numerical Schlieren picture of a simulated <strong>detonation</strong><br />
<strong>in</strong> a <strong>H2</strong>-<strong>N2O</strong>-<strong>Ar</strong> mixture. Initial conditions: Φ =<br />
1; X<strong>Ar</strong> =0.4;P1 = 10.1 kPa; T1 = 297 K.<br />
The <strong>numerical</strong> soot foils are used to estimate the <strong>cell</strong><br />
size. The mean <strong>cell</strong> size <strong>in</strong> the simulations presented<br />
<strong>in</strong> Figure 8 <strong>and</strong> Figure 9 is 9 mm, that is almost<br />
two times smaller than the experimental one. As the<br />
velocity of the simulated <strong>detonation</strong> is 50 m/s higher<br />
than <strong>in</strong> the experiment, it was decided to adjust the<br />
propagation velocity <strong>in</strong> the simulation. The desired<br />
effect can be achieved by modify<strong>in</strong>g the enthalpy<br />
of formation of <strong>H2</strong>O, the most important oxidation<br />
product. Based on computational tests, the modified<br />
enthalpy of formation must be -221.1 kJ/mol <strong>in</strong>stead<br />
of the st<strong>and</strong>ard value of -241.8 kJ/mol. Additionnal<br />
simulations have been performed <strong>and</strong> a mean <strong>cell</strong><br />
size of 18 mm was derived, perfectly match<strong>in</strong>g the<br />
experimental data. Figure 10 sums up the results<br />
of the different <strong>numerical</strong> simulations <strong>and</strong> compares<br />
them with the experimental results <strong>and</strong> Ng’s correlation.<br />
5<br />
Figure 9: Example of <strong>numerical</strong> soot foil obta<strong>in</strong>ed <strong>in</strong> a<br />
<strong>H2</strong>-<strong>N2O</strong>-<strong>Ar</strong> mixture. Initial conditions: Φ = 1; X<strong>Ar</strong> =<br />
0.4; P1 = 10.1 kPa; T1 = 297 K.<br />
λ (mm)<br />
100<br />
10<br />
<strong>Experimental</strong> data<br />
λnum-adjusted<br />
λnum<br />
λNg<br />
1<br />
Equivalence ratio<br />
Figure 10: Dependence of the <strong>cell</strong> size on the equivalence<br />
ratio <strong>in</strong> <strong>H2</strong>-<strong>N2O</strong>-<strong>Ar</strong> <strong>mixtures</strong>: comparison between experimental<br />
data <strong>and</strong> different predictions. Initial conditions:<br />
Φ = 0.3-2.5; X<strong>Ar</strong> =0.4;P1 =10kPa;T1 = 295 K.<br />
Theoretically, the most reliable method for <strong>cell</strong> size<br />
prediction would consist <strong>in</strong> deriv<strong>in</strong>g the <strong>detonation</strong><br />
<strong>cell</strong> size from <strong>numerical</strong> simulation. In practice, such<br />
simulations are very costly <strong>and</strong> require a drastic<br />
reduction of the chemical k<strong>in</strong>etics so that it is often<br />
taken <strong>in</strong>to account as a s<strong>in</strong>gle irreversible step.<br />
In <strong>detonation</strong> waves, <strong>in</strong>stabilities result from the<br />
coupl<strong>in</strong>g between chemistry <strong>and</strong> gasdynamics. Consequently,<br />
it is not surpris<strong>in</strong>g that the use of a s<strong>in</strong>gle<br />
reaction results <strong>in</strong> poor <strong>cell</strong> size prediction. In the<br />
present study, although the detailed scheme has been<br />
strongly reduced, the chemical mechanism consists<br />
of 5 reversible reactions. The <strong>cell</strong> size predicted<br />
us<strong>in</strong>g this approach is with<strong>in</strong> the experimental range<br />
of measurement. However, the obta<strong>in</strong>ed value is