Biochemical components of blood in normal and pathological ...
Biochemical components of blood in normal and pathological ...
Biochemical components of blood in normal and pathological ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
BIOCHEMICAL COMPONENTS OF BLOOD IN NORMAL AND<br />
PATHOLOGICAL CONDITIONS. BLOOD PROTEINS. NON-PROTEIN<br />
NITROGENOUS CONTAINING AND NON- NITROGENOUS CONTAINING<br />
COMPONENTS OF BLOOD.<br />
Blood is a liquid tissue. Suspended <strong>in</strong> the watery plasma are seven types <strong>of</strong> cells<br />
<strong>and</strong> cell fragments.<br />
red <strong>blood</strong> cells (RBCs) or erythrocytes<br />
platelets or thrombocytes<br />
five k<strong>in</strong>ds <strong>of</strong> white <strong>blood</strong> cells (WBCs) or leukocytes<br />
o Three k<strong>in</strong>ds <strong>of</strong> granulocytes<br />
neutrophils<br />
eos<strong>in</strong>ophils<br />
basophils<br />
o Two k<strong>in</strong>ds <strong>of</strong> leukocytes without granules <strong>in</strong> their cytoplasm<br />
lymphocytes<br />
monocytes<br />
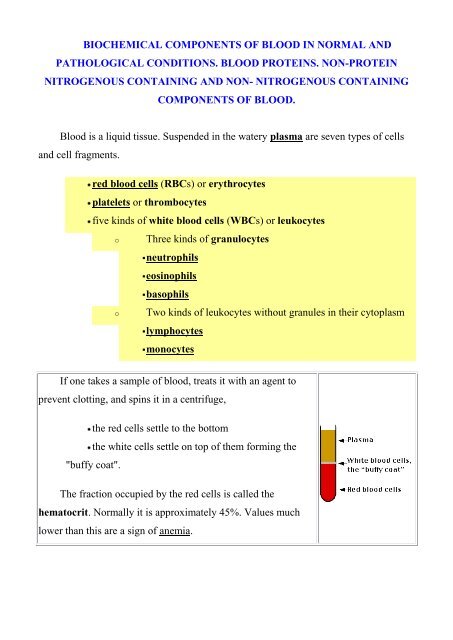
If one takes a sample <strong>of</strong> <strong>blood</strong>, treats it with an agent to<br />
prevent clott<strong>in</strong>g, <strong>and</strong> sp<strong>in</strong>s it <strong>in</strong> a centrifuge,<br />
the red cells settle to the bottom<br />
the white cells settle on top <strong>of</strong> them form<strong>in</strong>g the<br />
"buffy coat".<br />
The fraction occupied by the red cells is called the<br />
hematocrit. Normally it is approximately 45%. Values much<br />
lower than this are a sign <strong>of</strong> anemia.
Biological functions <strong>of</strong> the <strong>blood</strong><br />
The <strong>blood</strong> is the most specialized fluid tissue which circulates <strong>in</strong> vascular system<br />
<strong>and</strong> together with lymph <strong>and</strong> <strong>in</strong>tercellular space compounds an <strong>in</strong>ternal environment <strong>of</strong><br />
an organism.<br />
The <strong>blood</strong> executes such functions:<br />
1. Transport <strong>of</strong> gases – oxygen from lungs is carried to tissues <strong>and</strong> carbon dioxide<br />
from tissues to lungs.<br />
2. Transport <strong>of</strong> nutrients to all cells <strong>of</strong> organism (glucose, am<strong>in</strong>o acids, fatty acids,<br />
vitam<strong>in</strong>s, ketone bodies, trace substances <strong>and</strong> others). Substances such as urea, uric<br />
acid, bilirub<strong>in</strong> <strong>and</strong> creat<strong>in</strong><strong>in</strong>e are taken away from the different organs for ultimate<br />
excretion.<br />
3. Regulatory or hormonal function – hormones are secreted <strong>in</strong> to <strong>blood</strong> <strong>and</strong> they<br />
are transported by <strong>blood</strong> to their target cells.<br />
4. Thermoregulation function - an exchange <strong>of</strong> heat between tissues <strong>and</strong> <strong>blood</strong>.<br />
5. Osmotic function- susta<strong>in</strong>s osmotic pressure <strong>in</strong> vessels.<br />
6. Protective function- by the phagocytic action <strong>of</strong> leucocytes <strong>and</strong> by the actions <strong>of</strong><br />
antibodies, the <strong>blood</strong> provides the most important defense mechanism.<br />
7. Detoxification function - neutralization <strong>of</strong> toxic substances which is connected<br />
with their decomposition by the help <strong>of</strong> <strong>blood</strong> enzymes.<br />
Blood performs two major functions:<br />
transport through the body <strong>of</strong><br />
o oxygen <strong>and</strong> carbon dioxide<br />
o food molecules (glucose, lipids, am<strong>in</strong>o acids)<br />
o ions (e.g., Na + , Ca 2+ , HCO3 − )<br />
o wastes (e.g., urea)<br />
o hormones<br />
o heat<br />
defense <strong>of</strong> the body aga<strong>in</strong>st <strong>in</strong>fections <strong>and</strong> other foreign materials. All the<br />
WBCs participate <strong>in</strong> these defenses.
The formation <strong>of</strong> <strong>blood</strong> cells (cell types <strong>and</strong> acronyms are def<strong>in</strong>ed below)<br />
All the various types <strong>of</strong> <strong>blood</strong> cells<br />
human!).<br />
are produced <strong>in</strong> the bone marrow (some 10 11 <strong>of</strong> them each day <strong>in</strong> an adult<br />
arise from a s<strong>in</strong>gle type <strong>of</strong> cell called a hematopoietic stem cell — an<br />
"adult" multipotent stem cell.<br />
These stem cells<br />
are very rare (only about one <strong>in</strong> 10,000 bone marrow cells);<br />
are attached (probably by adherens junctions) to osteoblasts l<strong>in</strong><strong>in</strong>g the<br />
<strong>in</strong>ner surface <strong>of</strong> bone cavities;<br />
express a cell-surface prote<strong>in</strong> designated CD34;<br />
produce, by mitosis, two k<strong>in</strong>ds <strong>of</strong> progeny:
o more stem cells (A mouse that has had all its <strong>blood</strong> stem cells<br />
killed by a lethal dose <strong>of</strong> radiation can be saved by the <strong>in</strong>jection <strong>of</strong> a s<strong>in</strong>gle<br />
liv<strong>in</strong>g stem cell!).<br />
o cells that beg<strong>in</strong> to differentiate along the paths lead<strong>in</strong>g to the<br />
various k<strong>in</strong>ds <strong>of</strong> <strong>blood</strong> cells.<br />
Which path is taken is regulated by<br />
the need for more <strong>of</strong> that type <strong>of</strong> <strong>blood</strong> cell which is, <strong>in</strong> turn, controlled by<br />
appropriate cytok<strong>in</strong>es <strong>and</strong>/or hormones.<br />
Examples:<br />
Interleuk<strong>in</strong>-7 (IL-7) is the major cytok<strong>in</strong>e <strong>in</strong> stimulat<strong>in</strong>g bone marrow<br />
stem cells to start down the path lead<strong>in</strong>g to the various lymphocytes (mostly B<br />
cells <strong>and</strong> T cells).<br />
Erythropoiet<strong>in</strong> (EPO), produced by the kidneys, enhances the production<br />
<strong>of</strong> red <strong>blood</strong> cells (RBCs).<br />
Thrombopoiet<strong>in</strong> (TPO), assisted by Interleuk<strong>in</strong>-11 (IL-11), stimulates the<br />
production <strong>of</strong> megakaryocytes. Their fragmentation produces platelets.<br />
Granulocyte-macrophage colony-stimulat<strong>in</strong>g factor (GM-CSF), as its<br />
name suggests, sends cells down the path lead<strong>in</strong>g to both those cell types. In due<br />
course, one path or the other is taken.<br />
o Under the <strong>in</strong>fluence <strong>of</strong> granulocyte colony-stimulat<strong>in</strong>g<br />
factor (G-CSF), they differentiate <strong>in</strong>to neutrophils.<br />
o Further stimulated by <strong>in</strong>terleuk<strong>in</strong>-5 (IL-5) they develop <strong>in</strong>to<br />
eos<strong>in</strong>ophils.<br />
o Interleuk<strong>in</strong>-3 (IL-3) participates <strong>in</strong> the differentiation <strong>of</strong> most <strong>of</strong> the<br />
white <strong>blood</strong> cells but plays a particularly prom<strong>in</strong>ent role <strong>in</strong> the formation <strong>of</strong><br />
basophils (responsible for some allergies).
o Stimulated by macrophage colony-stimulat<strong>in</strong>g factor (M-CSF) the<br />
granulocyte/macrophage progenitor cells differentiate <strong>in</strong>to monocytes,<br />
macrophages, <strong>and</strong> dendritic cells (DCs).<br />
Biological chemistry <strong>of</strong> <strong>blood</strong> cells<br />
Two types <strong>of</strong> <strong>blood</strong> cells can be dist<strong>in</strong>guished - white <strong>and</strong> red <strong>blood</strong> cells. White<br />
<strong>blood</strong> cells are called leucocytes. Their quantity <strong>in</strong> adult is 4-9 x 10 9 /L.<br />
Red <strong>blood</strong> cells are called erythrocytes. Their quantity <strong>in</strong> peripheral <strong>blood</strong> is 4,5-5<br />
x 10 12 /L. Besides that, there are also thrombocytes or platelets <strong>in</strong> <strong>blood</strong>.<br />
White Blood Cells (leukocytes)<br />
Leucocytes (white <strong>blood</strong> cells) protect an organism from microorganisms, viruses<br />
<strong>and</strong> foreign substances, that provides the immune status <strong>of</strong> an organism.<br />
1:700),<br />
are much less numerous than red (the ratio between the two is around<br />
have nuclei,<br />
participate <strong>in</strong> protect<strong>in</strong>g the body from <strong>in</strong>fection,<br />
consist <strong>of</strong> lymphocytes <strong>and</strong> monocytes with relatively clear cytoplasm,<br />
<strong>and</strong> three types <strong>of</strong> granulocytes, whose cytoplasm is filled with granules.<br />
Leucocytes are divided <strong>in</strong>to two groups: Granulocytes <strong>and</strong> agranulocytes.<br />
Granulocytes consist <strong>of</strong> neutrophils, eos<strong>in</strong>ophils <strong>and</strong> basophils. Agranulocytes consist<br />
<strong>of</strong> monocytes <strong>and</strong> lymphocytes.<br />
http://www.youtube.com/watch?v=8ytkFqAMoa8<br />
http://www.youtube.com/watch?v=ce0Xndms1bc<br />
Neutrophils
Neutrophils comprise <strong>of</strong> 60-70 % from all leucocytes. Their ma<strong>in</strong> function is to<br />
protect organisms from microorganisms <strong>and</strong> viruses. Neutrophils have segmented<br />
nucleus, endoplasmic reticulum (underdeveloped) which does not conta<strong>in</strong> ribosomes,<br />
<strong>in</strong>sufficient amount <strong>of</strong> mitochondria, well-developed Golgi apparatus <strong>and</strong> hundreds <strong>of</strong><br />
different vesicles which conta<strong>in</strong> peroxidases <strong>and</strong> hydrolases. Optimum condition for<br />
their activity is acidic pH. There are also small vesicles which conta<strong>in</strong> alkal<strong>in</strong>e<br />
phosphatases, lysozymes, lactopher<strong>in</strong>s <strong>and</strong> prote<strong>in</strong>s <strong>of</strong> cationic orig<strong>in</strong>.<br />
Glucose is the ma<strong>in</strong> source <strong>of</strong> energy for neutrophils. It is directly utilized or<br />
converted <strong>in</strong>to glycogen. 90 % <strong>of</strong> energy is formed <strong>in</strong> glycolysis, a small amount <strong>of</strong><br />
glucose is converted <strong>in</strong> pentosophosphate pathway. Activation <strong>of</strong> proteolysis dur<strong>in</strong>g<br />
phagocytosis as well as reduction <strong>of</strong> phosphatidic acid <strong>and</strong> phosphoglycerols are also<br />
observed. The englobement is accompanied by <strong>in</strong>tensify<strong>in</strong>g <strong>of</strong> a glycolysis <strong>and</strong><br />
pentosophosphate pathway. But especially <strong>in</strong>tensity <strong>of</strong> absorption <strong>of</strong> oxygen for<br />
neutrophils - so-called flashout <strong>of</strong> respiration grows. Absorbed oxygen is spent for<br />
formation <strong>of</strong> its fissile forms that is carried out with participation enzymes:<br />
peroxide<br />
1. NADP*Н -OXYDASE catalyzes formation <strong>of</strong> super oxide anion<br />
2. An enzyme NADH- OXYDASE is responsible for formation <strong>of</strong> hydrogen<br />
3. Мyeloperoxydase catalyzes formation <strong>of</strong> hypochloric acid from chloride <strong>and</strong><br />
hydrogen peroxide<br />
Neutrophils are motile phagocyte cells that play a key role <strong>in</strong> acute <strong>in</strong>flammation.<br />
When bacteria enter tissues, a number <strong>of</strong> phenomena occur that are collectively known<br />
as acute <strong>in</strong>flammatory response. When neutrophils <strong>and</strong> other phagocyte cells engulf<br />
bacteria, they exhibit a rapid <strong>in</strong>crease <strong>in</strong> oxygen consumption known as the respiratory<br />
burst. This phenomenon reflects the rapid utilization <strong>of</strong> oxygen (follow<strong>in</strong>g a lag <strong>of</strong> 15-<br />
60 seconds) <strong>and</strong> production from it <strong>of</strong> large amounts <strong>of</strong> reactive derivates, such as O2 - ,<br />
H2O2, OH . <strong>and</strong> OCl - (hypochlorite ion). Some <strong>of</strong> these products are potent microbicidal<br />
agents. The electron transport cha<strong>in</strong> system responsible for the respiratory burst<br />
conta<strong>in</strong>s several <strong>components</strong>, <strong>in</strong>clud<strong>in</strong>g a flavoprote<strong>in</strong> NADPH:O2-oxidoreductase<br />
(<strong>of</strong>ten called NADPH-oxidase) <strong>and</strong> a b-type cytochrome.
emnants by phagocytosis.<br />
The most abundant <strong>of</strong> the WBCs. This<br />
photomicrograph shows a s<strong>in</strong>gle neutrophil<br />
surrounded by red <strong>blood</strong> cells.<br />
Neutrophils squeeze through the capillary<br />
walls <strong>and</strong> <strong>in</strong>to <strong>in</strong>fected tissue where they kill the<br />
<strong>in</strong>vaders (e.g., bacteria) <strong>and</strong> then engulf the<br />
This is a never-end<strong>in</strong>g task, even <strong>in</strong> healthy people: Our throat, nasal passages, <strong>and</strong><br />
colon harbor vast numbers <strong>of</strong> bacteria. Most <strong>of</strong> these are commensals, <strong>and</strong> do us no<br />
harm. But that is because neutrophils keep them <strong>in</strong> check.<br />
However,<br />
heavy doses <strong>of</strong> radiation<br />
chemotherapy<br />
<strong>and</strong> many other forms <strong>of</strong> stress<br />
can reduce the numbers <strong>of</strong> neutrophils so that formerly harmless bacteria beg<strong>in</strong> to<br />
proliferate. The result<strong>in</strong>g opportunistic <strong>in</strong>fection can be life-threaten<strong>in</strong>g.<br />
http://www.youtube.com/watch?v=EpC6G_DGqkI&feature=related<br />
Some important enzymes <strong>and</strong> prote<strong>in</strong>s <strong>of</strong> neutrophilis.<br />
Myeloperoxidase (MPO). Catalyzed follow<strong>in</strong>g reaction:<br />
H2O2 + X - (halide) + H +<br />
HOX=hypochlorous acid)<br />
HOX + H2O (where X - = Cl - , Br - , I - or SCN - ;<br />
HOCl, the active <strong>in</strong>gredient <strong>of</strong> household liquid bleach, is a powerful oxidant <strong>and</strong><br />
is highly microbicidial. When applied to <strong>normal</strong> tissues, its potential for caus<strong>in</strong>g<br />
damage is dim<strong>in</strong>ished because it reacts with primary or secondary am<strong>in</strong>es present <strong>in</strong><br />
neutrophils <strong>and</strong> tissues to produce various nitrogen-chlor<strong>in</strong>e (N-Cl) derivates; these<br />
chloram<strong>in</strong>es are also oxidants, although less powerful than HOCl, <strong>and</strong> act as
microbicidial agents (eg, <strong>in</strong> steriliz<strong>in</strong>g wounds) without caus<strong>in</strong>g tissue damage.<br />
Responsible for the green color <strong>of</strong> pus.<br />
NADPH-oxidase.<br />
2O2 + NADPH 2O2 - + NADP + H +<br />
Key component <strong>of</strong> the respiratory burst. Deficiency may be observed <strong>in</strong> chronic<br />
granulomatous disease.<br />
Lysozyme.<br />
Hydrolyzes l<strong>in</strong>k between N-acetylmuramic acid <strong>and</strong> N-acetyl-D-glucosam<strong>in</strong>e<br />
found <strong>in</strong> certa<strong>in</strong> bacterial cell walls. Abundant <strong>in</strong> macrophages.<br />
Defens<strong>in</strong>s.<br />
Basic antibiotic peptides <strong>of</strong> 29-33 am<strong>in</strong>o acids. Apparently kill bacteria by caus<strong>in</strong>g<br />
membrane damage.<br />
Lact<strong>of</strong>err<strong>in</strong>.<br />
Iron-b<strong>in</strong>d<strong>in</strong>g prote<strong>in</strong>. May <strong>in</strong>hibit growth <strong>of</strong> certa<strong>in</strong> bacteria by b<strong>in</strong>d<strong>in</strong>g iron <strong>and</strong><br />
may be <strong>in</strong>volved <strong>in</strong> regulation <strong>of</strong> proliferation <strong>of</strong> myeloid cells.<br />
Neutrophils conta<strong>in</strong> a number <strong>of</strong> prote<strong>in</strong>ases (elastase, collagenase, gelat<strong>in</strong>ase,<br />
catheps<strong>in</strong> G, plasm<strong>in</strong>ogen activator) that can hydrolyze elast<strong>in</strong>, various types <strong>of</strong><br />
collagens, <strong>and</strong> other prote<strong>in</strong>s present <strong>in</strong> the extracellular matrix. Such enzymatic action,<br />
if allowed to proceed unopposed, can result <strong>in</strong> serious damage to tissues. Most <strong>of</strong> these<br />
prote<strong>in</strong>ases are lysosomal enzymes <strong>and</strong> exist ma<strong>in</strong>ly as <strong>in</strong>active precursors <strong>in</strong> <strong>normal</strong><br />
neutrophils. Small amounts <strong>of</strong> these enzymes are released <strong>in</strong>to <strong>normal</strong> tissues, with the<br />
amounts <strong>in</strong>creas<strong>in</strong>g markedly dur<strong>in</strong>g <strong>in</strong>flammation. The activities <strong>of</strong> elastase <strong>and</strong> other<br />
prote<strong>in</strong>ases are <strong>normal</strong>ly kept <strong>in</strong> check by a number <strong>of</strong> antiprote<strong>in</strong>ases ( 1-<br />
Antiprote<strong>in</strong>ase, 2-Macroglobul<strong>in</strong>, Secretory leukoprote<strong>in</strong>ase <strong>in</strong>hibitor, 1-<br />
Antichymotryps<strong>in</strong>, Plasm<strong>in</strong>ogen activator <strong>in</strong>hibitor-1, Tissue <strong>in</strong>hibitor <strong>of</strong><br />
metalloprote<strong>in</strong>ase) present <strong>in</strong> plasma <strong>and</strong> the extracellular fluid.<br />
Basophiles<br />
Basophiles make up 1-5% <strong>of</strong> all <strong>blood</strong> leukocytes. They are actively formed <strong>in</strong><br />
the bone marrow dur<strong>in</strong>g allergy. Basophiles take part <strong>in</strong> the allergic reactions, <strong>in</strong> the
lood coagulation <strong>and</strong> <strong>in</strong>travascular lipolysis. They have the prote<strong>in</strong> synthesis<br />
mechanism, which works due to the biological oxidation energy . They synthesize<br />
the mediators <strong>of</strong> allergic reactions – histam<strong>in</strong>e <strong>and</strong> seroton<strong>in</strong>, which dur<strong>in</strong>g allergy<br />
cause local <strong>in</strong>flammation. Hepar<strong>in</strong>, which is formed <strong>in</strong> the basophiles, prevents the<br />
<strong>blood</strong> coagulation <strong>and</strong> activates <strong>in</strong>travascular lipoprote<strong>in</strong> lipase, which splits<br />
triacylglycer<strong>in</strong>.<br />
The number <strong>of</strong> basophils also <strong>in</strong>creases dur<strong>in</strong>g <strong>in</strong>fection. Basophils leave the <strong>blood</strong><br />
<strong>and</strong> accumulate at the site <strong>of</strong> <strong>in</strong>fection or other <strong>in</strong>flammation. There they discharge the<br />
contents <strong>of</strong> their granules, releas<strong>in</strong>g a variety <strong>of</strong> mediators such as:<br />
histam<strong>in</strong>e<br />
seroton<strong>in</strong><br />
prostagl<strong>and</strong><strong>in</strong>s <strong>and</strong> leukotrienes<br />
which <strong>in</strong>crease the <strong>blood</strong> flow to the area <strong>and</strong> <strong>in</strong> other ways add to the<br />
<strong>in</strong>flammatory process. The mediators released by basophils also play an important part<br />
<strong>in</strong> some allergic responses such as<br />
hay fever <strong>and</strong><br />
an anaphylactic response to <strong>in</strong>sect st<strong>in</strong>gs.<br />
Eos<strong>in</strong>ophiles<br />
They make up 3-6% <strong>of</strong> all leukocytes. Eos<strong>in</strong>ophiles as well as neutrophiles<br />
defend the cells from microorganisms, they conta<strong>in</strong> myeloperoxidase, lysosomal<br />
hydrolases. About the relations <strong>of</strong> eos<strong>in</strong>ophiles with testifies the growth <strong>of</strong> their<br />
amount dur<strong>in</strong>g the sensitization <strong>of</strong> organism, i.e. dur<strong>in</strong>g bronchial asthma,<br />
helm<strong>in</strong>thiasis. They are able to pile <strong>and</strong> splits histam<strong>in</strong>e, ―to dissolve‖ thrombus with<br />
the participation <strong>of</strong> plasm<strong>in</strong>ogen <strong>and</strong> bradyk<strong>in</strong><strong>in</strong>-k<strong>in</strong><strong>in</strong>ase.<br />
Monocytes
They are formed <strong>in</strong> the bone marrow. They make up 4-8% <strong>of</strong> all leukocytes.<br />
Accord<strong>in</strong>g to the function they are called macrophages. Tissue macrophages derive<br />
from <strong>blood</strong> monocytes. Depend<strong>in</strong>g on their position they are called: <strong>in</strong> the liver –<br />
reticuloendotheliocytes, <strong>in</strong> the lungs - alveolar macrophages, <strong>in</strong> the <strong>in</strong>termediate<br />
substance <strong>of</strong> connective tissue – histocytes etc. Monocytes are characterized by a<br />
wide set <strong>of</strong> lysosomal enzymes with the optimum activity <strong>in</strong> the acidic condition.<br />
The major functions <strong>of</strong> monocytes <strong>and</strong> macrophages are endocytosis <strong>and</strong><br />
phagocytosis.<br />
Lymphocytes<br />
The amount – 20-25%, are formed <strong>in</strong> the lymphoid tissue or thymus, play<br />
important role <strong>in</strong> the formation <strong>of</strong> humoral <strong>and</strong> cellular immunity. Lymphocytes have<br />
powerful system <strong>of</strong> synthesis <strong>of</strong> antibody prote<strong>in</strong>s, energy is majorily perta<strong>in</strong>ed due<br />
to glycolysis, rarely – by aerobic way.<br />
http://www.youtube.com/watch?v=cD_uAGPBfQQ&feature=related<br />
There are several k<strong>in</strong>ds <strong>of</strong> lymphocytes (although they all look alike under the<br />
microscope), each with different functions to perform . The most common types <strong>of</strong><br />
lymphocytes are<br />
B lymphocytes ("B cells"). These are responsible for mak<strong>in</strong>g antibodies.<br />
T lymphocytes ("T cells"). There are several subsets <strong>of</strong> these:<br />
o <strong>in</strong>flammatory T cells that recruit macrophages <strong>and</strong><br />
neutrophils to the site <strong>of</strong> <strong>in</strong>fection or other tissue damage<br />
o cytotoxic T lymphocytes (CTLs) that kill virus-<strong>in</strong>fected <strong>and</strong>,<br />
perhaps, tumor cells<br />
cells<br />
o helper T cells that enhance the production <strong>of</strong> antibodies by B
Monocytes<br />
cont<strong>in</strong>ue to divide by mitosis;<br />
Although bone marrow is the ultimate source <strong>of</strong><br />
lymphocytes, the lymphocytes that will become T<br />
cells migrate from the bone marrow to the thymus<br />
where they mature. Both B cells <strong>and</strong> T cells also take<br />
up residence <strong>in</strong> lymph nodes, the spleen <strong>and</strong> other<br />
tissues where they<br />
mature <strong>in</strong>to fully functional cells.<br />
encounter antigens;<br />
Monocytes leave the <strong>blood</strong> <strong>and</strong> become macrophages <strong>and</strong> dendritic cells.<br />
This scann<strong>in</strong>g electron micrograph (courtesy <strong>of</strong> Drs. Jan M. Orenste<strong>in</strong> <strong>and</strong> Emma<br />
Shelton) shows a s<strong>in</strong>gle macrophage surrounded by several lymphocytes.<br />
Macrophages are large, phagocytic cells that engulf<br />
foreign material (antigens) that enter the body<br />
dead <strong>and</strong> dy<strong>in</strong>g cells <strong>of</strong> the body.<br />
Thrombocytes (<strong>blood</strong> platelets)<br />
Platelets are cell fragments produced from megakaryocytes.<br />
Blood <strong>normal</strong>ly conta<strong>in</strong>s 150,000–350,000 per microliter (µl) or cubic millimeter<br />
(mm 3 ). This number is <strong>normal</strong>ly ma<strong>in</strong>ta<strong>in</strong>ed by a homeostatic (negative-feedback)<br />
mechanism .<br />
The amount – less than 1%, they play the ma<strong>in</strong> role <strong>in</strong> the process <strong>of</strong><br />
hemostasis. They are formed as a result <strong>of</strong> dis<strong>in</strong>tegration <strong>of</strong> megakaryocytes <strong>in</strong> the<br />
bone marrow. Their –life-time is 7-9 days. In spite <strong>of</strong> the fact that thrombocytes<br />
have no nucleus, they are able to perform practically all functions <strong>of</strong> the cell, besides<br />
DNA synthesis.
If this value should drop much below 50,000/µl, there is a danger <strong>of</strong> uncontrolled<br />
bleed<strong>in</strong>g because <strong>of</strong> the essential role that platelets have <strong>in</strong> <strong>blood</strong> clott<strong>in</strong>g.<br />
Some causes:<br />
certa<strong>in</strong> drugs <strong>and</strong> herbal remedies;<br />
autoimmunity.<br />
When <strong>blood</strong> vessels are cut or damaged, the loss <strong>of</strong> <strong>blood</strong> from the system must be<br />
stopped before shock <strong>and</strong> possible death occur. This is accomplished by solidification<br />
<strong>of</strong> the <strong>blood</strong>, a process called coagulation or clott<strong>in</strong>g.<br />
A <strong>blood</strong> clot consists <strong>of</strong><br />
a plug <strong>of</strong> platelets enmeshed <strong>in</strong> a<br />
network <strong>of</strong> <strong>in</strong>soluble fibr<strong>in</strong> molecules.<br />
The most numerous type <strong>in</strong> the <strong>blood</strong>.<br />
Red Blood Cells (erythrocytes)<br />
Women average about 4.8 million <strong>of</strong> these cells per cubic millimeter (mm 3 ;<br />
which is the same as a microliter [µl]) <strong>of</strong> <strong>blood</strong>.<br />
Men average about 5.4 x 10 6 per µl.<br />
These values can vary over quite a range depend<strong>in</strong>g on such factors as<br />
health <strong>and</strong> altitude. (Peruvians liv<strong>in</strong>g at 18,000 feet may have as many as 8.3 x<br />
10 6 RBCs per µl.)<br />
RBC precursors mature <strong>in</strong> the bone marrow closely attached to a macrophage.<br />
They manufacture hemoglob<strong>in</strong> until it accounts for some 90% <strong>of</strong> the dry<br />
weight <strong>of</strong> the cell.<br />
exosomes.<br />
The nucleus is squeezed out <strong>of</strong> the cell <strong>and</strong> is <strong>in</strong>gested by the macrophage.<br />
No-longer-needed prote<strong>in</strong>s are expelled from the cell <strong>in</strong> vesicles called
Human <strong>blood</strong> conta<strong>in</strong>s 25 trillion <strong>of</strong> erythrocytes.<br />
Their ma<strong>in</strong> function – transportation <strong>of</strong> O2 <strong>and</strong> CO2 – they<br />
perform due to the fact that they conta<strong>in</strong> 34% <strong>of</strong><br />
hemoglob<strong>in</strong>, <strong>and</strong> per dry cells mass – 95%. The total<br />
amount <strong>of</strong> hemoglob<strong>in</strong> <strong>in</strong> the <strong>blood</strong> equals 130-160 g/l. In<br />
the process <strong>of</strong> erythropoesis the preced<strong>in</strong>g cells decrease<br />
their size. Their nuclei at the end <strong>of</strong> the process are ru<strong>in</strong>ed<br />
<strong>and</strong> pushed out <strong>of</strong> the cells. 90% <strong>of</strong> glucose <strong>in</strong> the<br />
erythrocytes is decomposed <strong>in</strong> the process <strong>of</strong> glycolysis<br />
<strong>and</strong> 10% - by pentose-phosphate way. There are noted<br />
congenital defects <strong>of</strong> enzymes <strong>of</strong> these metabolic ways <strong>of</strong> erythrocytes. Dur<strong>in</strong>g this<br />
are usually observed hemolytic anemia <strong>and</strong> other structural <strong>and</strong> functional<br />
erythrocytes’ affections.<br />
This scann<strong>in</strong>g electron micrograph (courtesy <strong>of</strong> Dr. Marion J. Barnhart) shows the<br />
characteristic biconcave shape <strong>of</strong> red <strong>blood</strong> cells.<br />
Thus RBCs are term<strong>in</strong>ally differentiated; that is, they can never divide. They live<br />
about 120 days <strong>and</strong> then are <strong>in</strong>gested by phagocytic cells <strong>in</strong> the liver <strong>and</strong> spleen. Most<br />
<strong>of</strong> the iron <strong>in</strong> their hemoglob<strong>in</strong> is reclaimed for reuse. The rema<strong>in</strong>der <strong>of</strong> the heme<br />
portion <strong>of</strong> the molecule is degraded <strong>in</strong>to bile pigments <strong>and</strong> excreted by the liver. Some<br />
3 million RBCs die <strong>and</strong> are scavenged by the liver each second.<br />
Red <strong>blood</strong> cells are responsible for the transport <strong>of</strong> oxygen <strong>and</strong> carbon dioxide.<br />
Oxygen Transport<br />
In adult humans the hemoglob<strong>in</strong> (Hb) molecule<br />
consists <strong>of</strong> four polypeptides:
o two alpha (α) cha<strong>in</strong>s <strong>of</strong> 141 am<strong>in</strong>o acids <strong>and</strong><br />
o two beta (β) cha<strong>in</strong>s <strong>of</strong> 146 am<strong>in</strong>o acids<br />
One molecule <strong>of</strong> oxygen can b<strong>in</strong>d to each heme.<br />
Each <strong>of</strong> these is attached the<br />
prosthetic group heme.<br />
There is one atom <strong>of</strong> iron at<br />
the center <strong>of</strong> each heme.<br />
http://www.youtube.com/watch?v=WXOBJEXxNEo&feature=related<br />
The reaction is reversible.<br />
Under the conditions <strong>of</strong> lower temperature, higher pH, <strong>and</strong> <strong>in</strong>creased<br />
oxygen pressure <strong>in</strong> the capillaries <strong>of</strong> the lungs, the reaction proceeds to the right.<br />
The purple-red deoxygenated hemoglob<strong>in</strong> <strong>of</strong> the venous <strong>blood</strong> becomes the<br />
bright-red oxyhemoglob<strong>in</strong> <strong>of</strong> the arterial <strong>blood</strong>.<br />
Under the conditions <strong>of</strong> higher temperature, lower pH, <strong>and</strong> lower oxygen<br />
pressure <strong>in</strong> the tissues, the reverse reaction is promoted <strong>and</strong> oxyhemoglob<strong>in</strong> gives<br />
up its oxygen.<br />
Carbon Dioxide Transport<br />
Carbon dioxide (CO2) comb<strong>in</strong>es with water form<strong>in</strong>g carbonic acid, which<br />
dissociates <strong>in</strong>to a hydrogen ion (H + ) <strong>and</strong> a bicarbonate ions<br />
:<br />
CO2 + H2O ↔ H2CO3 ↔ H + + HCO3 −<br />
95% <strong>of</strong> the CO2 generated <strong>in</strong> the tissues is carried <strong>in</strong> the red <strong>blood</strong> cells:
It probably enters (<strong>and</strong> leaves) the cell by diffus<strong>in</strong>g through transmembrane<br />
channels <strong>in</strong> the plasma membrane. (One <strong>of</strong> the prote<strong>in</strong>s that forms the channel is<br />
the D antigen that is the most important factor <strong>in</strong> the Rh system <strong>of</strong> <strong>blood</strong><br />
groups.)<br />
Once <strong>in</strong>side, about one-half <strong>of</strong> the CO2 is directly bound to hemoglob<strong>in</strong> (at<br />
a site different from the one that b<strong>in</strong>ds oxygen).<br />
The rest is converted — follow<strong>in</strong>g the equation above — by the enzyme<br />
carbonic anhydrase <strong>in</strong>to<br />
o bicarbonate ions that diffuse back out <strong>in</strong>to the plasma <strong>and</strong><br />
o hydrogen ions (H + ) that b<strong>in</strong>d to the prote<strong>in</strong> portion <strong>of</strong> the<br />
hemoglob<strong>in</strong> (thus hav<strong>in</strong>g no effect on pH).<br />
Only about 5% <strong>of</strong> the CO2 generated <strong>in</strong> the tissues dissolves directly <strong>in</strong> the plasma.<br />
(A good th<strong>in</strong>g, too: if all the CO2 we make were carried this way, the pH <strong>of</strong> the <strong>blood</strong><br />
would drop from its <strong>normal</strong> 7.4 to an <strong>in</strong>stantly-fatal 4.5!)<br />
When the red cells reach the lungs, these reactions are reversed <strong>and</strong> CO2 is<br />
released to the air <strong>of</strong> the alveoli.<br />
Anemia<br />
Anemia is a shortage <strong>of</strong><br />
RBCs <strong>and</strong>/or<br />
the amount <strong>of</strong> hemoglob<strong>in</strong> <strong>in</strong> them.<br />
Anemia has many causes. One <strong>of</strong> the most common is an <strong>in</strong>adequate <strong>in</strong>take <strong>of</strong> iron<br />
<strong>in</strong> the diet.<br />
Blood Groups<br />
Red <strong>blood</strong> cells have surface antigens that differ between people <strong>and</strong> that create<br />
the so-called <strong>blood</strong> groups such as the ABO system <strong>and</strong> the Rh system.<br />
An Essay on Hemoglob<strong>in</strong> Structure <strong>and</strong> Function:
Biol 175 pp. 159 (1984)<br />
Figure 1 is a model <strong>of</strong> human deoxyhemoglob<strong>in</strong>. It was<br />
created <strong>in</strong> RasMol version 2.6 by Roger Sayle us<strong>in</strong>g the pdb<br />
coord<strong>in</strong>ates from the pdb file 4hhb. The 3D coord<strong>in</strong>ates<br />
were determed from x-ray crystallography by Fermi, G.,<br />
Perutz, M. F., Shaanan, B., Fourme, R.: The crystal structure<br />
<strong>of</strong> human deoxyhaemoglob<strong>in</strong> at 1.74 A resolution. J Mol<br />
Hemoglob<strong>in</strong> is the prote<strong>in</strong> that carries oxygen from the lungs to the tissues <strong>and</strong><br />
carries carbon dioxide from the tissues back to the lungs. In order to function most<br />
efficiently, hemoglob<strong>in</strong> needs to b<strong>in</strong>d to oxygen tightly <strong>in</strong> the oxygen-rich atmosphere<br />
<strong>of</strong> the lungs <strong>and</strong> be able to release oxygen rapidly <strong>in</strong> the relatively oxygen-poor<br />
environment <strong>of</strong> the tissues. It does this <strong>in</strong> a most elegant <strong>and</strong> <strong>in</strong>tricately coord<strong>in</strong>ated<br />
way. The story <strong>of</strong> hemoglob<strong>in</strong> is the prototype example <strong>of</strong> the relationship between<br />
structure <strong>and</strong> function <strong>of</strong> a prote<strong>in</strong> molecule.<br />
Hemoglob<strong>in</strong> Structure<br />
A hemoglob<strong>in</strong> molecule consists <strong>of</strong> four polypeptide cha<strong>in</strong>s: two alpha cha<strong>in</strong>s,<br />
each with 141 am<strong>in</strong>o acids <strong>and</strong> two beta cha<strong>in</strong>s, each with 146 am<strong>in</strong>o acids. The prote<strong>in</strong><br />
portion <strong>of</strong> each <strong>of</strong> these cha<strong>in</strong>s is called "glob<strong>in</strong>". The a <strong>and</strong> b glob<strong>in</strong> cha<strong>in</strong>s are very<br />
similar <strong>in</strong> structure. In this case, a <strong>and</strong> b refer to the two types <strong>of</strong> glob<strong>in</strong>. Students <strong>of</strong>ten<br />
confuse this with the concept <strong>of</strong> a helix <strong>and</strong> b sheet secondary structures. But, <strong>in</strong> fact,<br />
both the a <strong>and</strong> b glob<strong>in</strong> cha<strong>in</strong>s conta<strong>in</strong> primarily a helix secondary structure with no b<br />
sheets.<br />
Figure 2 is a close up view <strong>of</strong> one <strong>of</strong> the<br />
heme groups <strong>of</strong> the human a cha<strong>in</strong> from<br />
dexoyhemoglob<strong>in</strong>. In this view, the iron is
coord<strong>in</strong>ated by a histid<strong>in</strong>e side cha<strong>in</strong> from am<strong>in</strong>o acid 87 (shown <strong>in</strong> green.)<br />
Each a or b glob<strong>in</strong> cha<strong>in</strong> folds <strong>in</strong>to 8 a helical segments (A-H) which, <strong>in</strong> turn, fold<br />
to form globular tertiary structures that look roughly like sub-microscopic kidney<br />
beans. The folded helices form a pocket that holds the work<strong>in</strong>g part <strong>of</strong> each cha<strong>in</strong>, the<br />
heme.<br />
http://www.youtube.com/watch?v=eor6EK_JP40<br />
A heme group is a flat r<strong>in</strong>g molecule conta<strong>in</strong><strong>in</strong>g carbon, nitrogen <strong>and</strong> hydrogen<br />
atoms, with a s<strong>in</strong>gle Fe 2+ ion at the center. Without the iron, the r<strong>in</strong>g is called a<br />
porphyr<strong>in</strong>. In a heme molecule, the iron is held with<strong>in</strong> the flat plane by four nitrogen<br />
lig<strong>and</strong>s from the porphyr<strong>in</strong> r<strong>in</strong>g. The iron ion makes a fifth bond to a histid<strong>in</strong>e side<br />
cha<strong>in</strong> from one <strong>of</strong> the helices that form the heme pocket. This fifth coord<strong>in</strong>ation bond is<br />
to histid<strong>in</strong>e 87 <strong>in</strong> the human a cha<strong>in</strong> <strong>and</strong> histid<strong>in</strong>e 92 <strong>in</strong> the human b cha<strong>in</strong>. Both<br />
histid<strong>in</strong>e residues are part <strong>of</strong> the F helix <strong>in</strong> each glob<strong>in</strong> cha<strong>in</strong>. t<br />
The Bohr Effect<br />
The ability <strong>of</strong> hemoglob<strong>in</strong> to release oxygen, is affected by pH, CO2 <strong>and</strong> by<br />
the differences <strong>in</strong> the oxygen-rich environment <strong>of</strong> the lungs <strong>and</strong> the oxygen-poor<br />
environment <strong>of</strong> the tissues. The pH <strong>in</strong> the tissues is considerably lower (more<br />
acidic) than <strong>in</strong> the lungs. Protons are generated from the reaction between carbon<br />
dioxide <strong>and</strong> water to form bicarbonate:<br />
CO2 + H20 -----------------> HCO3 - + H +<br />
This <strong>in</strong>creased acidity serves a tw<strong>of</strong>old purpose. First, protons lower the<br />
aff<strong>in</strong>ity <strong>of</strong> hemoglob<strong>in</strong> for oxygen, allow<strong>in</strong>g easier release <strong>in</strong>to the tissues. As all<br />
four oxygens are released, hemoglob<strong>in</strong> b<strong>in</strong>ds to two protons. This helps to<br />
ma<strong>in</strong>ta<strong>in</strong> equilibrium towards the right side <strong>of</strong> the equation. This is known as the<br />
Bohr effect, <strong>and</strong> is vital <strong>in</strong> the removal <strong>of</strong> carbon dioxide as waste because CO2<br />
is <strong>in</strong>soluble <strong>in</strong> the <strong>blood</strong>stream. The bicarbonate ion is much more soluble, <strong>and</strong><br />
can thereby be transported back to the lungs after be<strong>in</strong>g bound to hemoglob<strong>in</strong>. If
hemoglob<strong>in</strong> couldn’t absorb the excess protons, the equilibrium would shift to<br />
the left, <strong>and</strong> carbon dioxide couldn’t be removed.<br />
In the lungs, this effect works <strong>in</strong> the reverse direction. In the presence <strong>of</strong> the<br />
high oxygen concentration <strong>in</strong> the lungs, the proton aff<strong>in</strong>ity decreases. As protons<br />
are shed, the reaction is driven to the left, <strong>and</strong> CO2 forms as an <strong>in</strong>soluble gas to<br />
be expelled from the lungs. The proton poor hemoglob<strong>in</strong> now has a greater<br />
aff<strong>in</strong>ity for oxygen, <strong>and</strong> the cycle cont<strong>in</strong>ues.<br />
Haemoglob<strong>in</strong> or hemoglob<strong>in</strong> (frequently abbreviated as Hb or Hgb) is the iron-<br />
conta<strong>in</strong><strong>in</strong>g oxygen-transport metalloprote<strong>in</strong> <strong>in</strong> the red <strong>blood</strong> cells <strong>of</strong> the <strong>blood</strong> <strong>in</strong><br />
vertebrates <strong>and</strong> other animals; <strong>in</strong> mammals the prote<strong>in</strong> makes up about 97% <strong>of</strong> the red<br />
cell’s dry content, <strong>and</strong> around 35% <strong>of</strong> the total content <strong>in</strong>clud<strong>in</strong>g water. Hemoglob<strong>in</strong><br />
transports oxygen from the lungs or gills to the rest <strong>of</strong> the body, such as to the muscles,<br />
where it releases the oxygen load. Hemoglob<strong>in</strong> also has a variety <strong>of</strong> other gas-transport<br />
<strong>and</strong> effect-modulation duties, which vary from species to species, <strong>and</strong> which <strong>in</strong><br />
<strong>in</strong>vertebrates may be quite diverse.<br />
The name hemoglob<strong>in</strong> is the concatenation <strong>of</strong> heme <strong>and</strong> glob<strong>in</strong>, reflect<strong>in</strong>g the fact<br />
that each subunit <strong>of</strong> hemoglob<strong>in</strong> is a globular prote<strong>in</strong> with an embedded heme (or<br />
haem) group; each heme group conta<strong>in</strong>s an iron atom, <strong>and</strong> this is responsible for the<br />
b<strong>in</strong>d<strong>in</strong>g <strong>of</strong> oxygen. The most common type <strong>of</strong> hemoglob<strong>in</strong> <strong>in</strong> mammals conta<strong>in</strong>s four<br />
such subunits, each with one heme group.<br />
Mutations <strong>in</strong> the genes for the hemoglob<strong>in</strong> prote<strong>in</strong> <strong>in</strong> humans result <strong>in</strong> a group <strong>of</strong><br />
hereditary diseases termed the hemoglob<strong>in</strong>opathies, the most common members <strong>of</strong><br />
which are sickle-cell disease <strong>and</strong> thalassemia. Historically <strong>in</strong> human medic<strong>in</strong>e, the<br />
hemoglob<strong>in</strong>opathy <strong>of</strong> sickle-cell disease was the first disease to be understood <strong>in</strong> its<br />
mechanism <strong>of</strong> dysfunction, completely down to the molecular level. However, not all <strong>of</strong><br />
such mutations produce disease states, <strong>and</strong> are formally recognized as hemoglob<strong>in</strong><br />
variants (not diseases). [1][2]
Hemoglob<strong>in</strong> (Hb) is synthesized <strong>in</strong> a complex series <strong>of</strong> steps. The heme portion is<br />
sythesized <strong>in</strong> both the the mitochondria <strong>and</strong> cytosol <strong>of</strong> the immature red <strong>blood</strong> cell,<br />
while the glob<strong>in</strong> prote<strong>in</strong> portions <strong>of</strong> the molecule are sythesized by ribosomes <strong>in</strong> the<br />
cytosol [3] . Production <strong>of</strong> Hb cont<strong>in</strong>ues <strong>in</strong> the cell throughout its early development from<br />
the proerythroblast to the reticulocyte <strong>in</strong> the bone marrow. At this po<strong>in</strong>t, the nucleus is<br />
lost <strong>in</strong> mammals, but not <strong>in</strong> birds <strong>and</strong> many other species. Even after the loss <strong>of</strong> the<br />
nucleus <strong>in</strong> mammals, however, residual ribosomal RNA allows further synthesis <strong>of</strong> Hb<br />
until the reticulocyte loses its RNA soon after enter<strong>in</strong>g the vasculature (this<br />
hemoglob<strong>in</strong>-synthetic RNA <strong>in</strong> fact gives the reticulocyte its reticulated appearance <strong>and</strong><br />
name).<br />
The empirical chemical formula <strong>of</strong> the most common human hemoglob<strong>in</strong> is<br />
C2952H4664N812O832S8Fe4, but as noted above, hemoglob<strong>in</strong>s vary widely across species,<br />
<strong>and</strong> even (through common mutations) slightly among subgroups <strong>of</strong> humans.<br />
In humans, the hemoglob<strong>in</strong> molecule is an assembly <strong>of</strong> four globular prote<strong>in</strong><br />
subunits. Each subunit is composed <strong>of</strong> a prote<strong>in</strong> cha<strong>in</strong> tightly associated with a non-<br />
prote<strong>in</strong> heme group. Each prote<strong>in</strong> cha<strong>in</strong> arranges <strong>in</strong>to a set <strong>of</strong> alpha-helix structural<br />
segments connected together <strong>in</strong> a glob<strong>in</strong> fold arrangement, so called because this<br />
arrangement is the same fold<strong>in</strong>g motif used <strong>in</strong> other heme/glob<strong>in</strong> prote<strong>in</strong>s such as<br />
myoglob<strong>in</strong>. [4][5] This fold<strong>in</strong>g pattern conta<strong>in</strong>s a pocket which strongly b<strong>in</strong>ds the heme<br />
group.<br />
A heme group consists <strong>of</strong> an iron (Fe) atom held <strong>in</strong> a heterocyclic r<strong>in</strong>g, known as a<br />
porphyr<strong>in</strong>. The iron atom, which is the site <strong>of</strong> oxygen b<strong>in</strong>d<strong>in</strong>g, bonds with the four<br />
nitrogens <strong>in</strong> the center <strong>of</strong> the r<strong>in</strong>g, which all lie <strong>in</strong> one plane. The iron is also bound<br />
strongly to the globular prote<strong>in</strong> via the imidazole r<strong>in</strong>g <strong>of</strong> a histid<strong>in</strong>e residue below the<br />
porphyr<strong>in</strong> r<strong>in</strong>g. A sixth position can reversibly b<strong>in</strong>d oxygen, complet<strong>in</strong>g the octahedral<br />
group <strong>of</strong> six lig<strong>and</strong>s. Oxygen b<strong>in</strong>ds <strong>in</strong> an "end-on bent" geometry where one oxygen<br />
atom b<strong>in</strong>ds Fe <strong>and</strong> the other protrudes at an angle. When oxygen is not bound, a very<br />
weakly bonded water molecule fills the site, form<strong>in</strong>g a distorted octahedron.
The iron atom may either be <strong>in</strong> the Fe 2+ or Fe 3+ state, but ferrihemoglob<strong>in</strong><br />
(methemoglob<strong>in</strong>) (Fe 3+ ) cannot b<strong>in</strong>d oxygen. In b<strong>in</strong>d<strong>in</strong>g, oxygen temporarily oxidizes<br />
Fe to (Fe 3+ ), so iron must exist <strong>in</strong> the +2 oxidation state <strong>in</strong> order to b<strong>in</strong>d oxygen. The<br />
body reactivates hemoglob<strong>in</strong> found <strong>in</strong> the <strong>in</strong>active (Fe 3+ ) state by reduc<strong>in</strong>g the iron<br />
center.<br />
In adult humans, the most common hemoglob<strong>in</strong> type is a tetramer (which conta<strong>in</strong>s<br />
4 subunit prote<strong>in</strong>s) called hemoglob<strong>in</strong> A, consist<strong>in</strong>g <strong>of</strong> two α <strong>and</strong> two β subunits non-<br />
covalently bound, each made <strong>of</strong> 141 <strong>and</strong> 146 am<strong>in</strong>o acid residues, respectively. This is<br />
denoted as α2β2. The subunits are structurally similar <strong>and</strong> about the same size. Each<br />
subunit has a molecular weight <strong>of</strong> about 17,000 daltons, for a total molecular weight <strong>of</strong><br />
the tetramer <strong>of</strong> about 68,000 daltons. Hemoglob<strong>in</strong> A is the most <strong>in</strong>tensively studied <strong>of</strong><br />
the hemoglob<strong>in</strong> molecules.<br />
The four polypeptide cha<strong>in</strong>s are bound to each other by salt bridges, hydrogen<br />
bonds, <strong>and</strong> hydrophobic <strong>in</strong>teractions. There are two k<strong>in</strong>ds <strong>of</strong> contacts between the α <strong>and</strong><br />
β cha<strong>in</strong>s: α1β1 <strong>and</strong> α1β2.<br />
Oxyhemoglob<strong>in</strong> is formed dur<strong>in</strong>g respiration when oxygen b<strong>in</strong>ds to the heme<br />
component <strong>of</strong> the prote<strong>in</strong> hemoglob<strong>in</strong> <strong>in</strong> red <strong>blood</strong> cells. This process occurs <strong>in</strong> the<br />
pulmonary capillaries adjacent to the alveoli <strong>of</strong> the lungs. The oxygen then travels<br />
through the <strong>blood</strong> stream to be dropped <strong>of</strong>f at cells where it is utilized <strong>in</strong> aerobic<br />
glycolysis <strong>and</strong> <strong>in</strong> the production <strong>of</strong> ATP by the process <strong>of</strong> oxidative phosphorylation. It<br />
doesn't however help to counteract a decrease <strong>in</strong> <strong>blood</strong> pH. Ventilation, or breath<strong>in</strong>g,<br />
may reverse this condition by removal <strong>of</strong> carbon dioxide, thus caus<strong>in</strong>g a shift up <strong>in</strong><br />
pH. [6]<br />
Deoxyhemoglob<strong>in</strong> is the form <strong>of</strong> hemoglob<strong>in</strong> without the bound oxygen. The<br />
absorption spectra <strong>of</strong> oxyhemoglob<strong>in</strong> <strong>and</strong> deoxyhemoglob<strong>in</strong> differ. The<br />
oxyhemoglob<strong>in</strong>e has significantly lower absorption <strong>of</strong> the 660 nm wavelength than<br />
deoxyhemoglob<strong>in</strong>, while at 940 nm its absorption is slightly higher. This difference is
used for measurement <strong>of</strong> the amount <strong>of</strong> oxygen <strong>in</strong> patient's <strong>blood</strong> by an <strong>in</strong>strument<br />
called pulse oximeter.<br />
Iron's oxidation state <strong>in</strong> oxyhemoglob<strong>in</strong><br />
The oxidation state <strong>of</strong> iron <strong>in</strong> hemoglob<strong>in</strong> is always +2. It does not change when<br />
oxygen b<strong>in</strong>ds to the deoxy- form.<br />
Assign<strong>in</strong>g oxygenated hemoglob<strong>in</strong>'s oxidation state is difficult because<br />
oxyhemoglob<strong>in</strong> is diamagnetic (no net unpaired electrons), but the low-energy electron<br />
configurations <strong>in</strong> both oxygen <strong>and</strong> iron are paramagnetic. Triplet oxygen, the lowest<br />
energy oxygen species, has two unpaired electrons <strong>in</strong> antibond<strong>in</strong>g π* molecular orbitals.<br />
Iron(II) tends to be <strong>in</strong> a high-sp<strong>in</strong> configuration where unpaired electrons exist <strong>in</strong> eg<br />
antibond<strong>in</strong>g orbitals. Iron(III) has an odd number <strong>of</strong> electrons <strong>and</strong> necessarily has
unpaired electrons. All <strong>of</strong> these molecules are paramagnetic (have unpaired electrons),<br />
not diamagnetic, so an un<strong>in</strong>tuitive distribution <strong>of</strong> electrons must exist to <strong>in</strong>duce<br />
diamagnetism.<br />
The three logical possibilities are:<br />
1) Low-sp<strong>in</strong> Fe 2+ b<strong>in</strong>ds to high-energy s<strong>in</strong>glet oxygen. Both low-sp<strong>in</strong> iron <strong>and</strong><br />
s<strong>in</strong>glet oxygen are diamagnetic.<br />
2) High-sp<strong>in</strong> Fe 3+ b<strong>in</strong>ds to .O2 - (the superoxide ion) <strong>and</strong> antiferromagnetism<br />
oppositely aligns the two unpaired electrons, giv<strong>in</strong>g diamagnetic properties.<br />
3) Low-sp<strong>in</strong> Fe 4+ b<strong>in</strong>ds to O2 2- . Both are diamagnetic.<br />
X-ray photoelectron spectroscopy suggests that iron has an oxidation state <strong>of</strong><br />
approximately 3.2 <strong>and</strong> <strong>in</strong>frared stretch<strong>in</strong>g frequencies <strong>of</strong> the O-O bond suggests a bond<br />
length fitt<strong>in</strong>g with superoxide. The correct oxidation state <strong>of</strong> iron is thus the +3 state<br />
with oxygen <strong>in</strong> the -1 state. The diamagnetism <strong>in</strong> this configuration arises from the<br />
unpaired electron on superoxide align<strong>in</strong>g antiferromagnetically <strong>in</strong> the opposite direction<br />
from the unpaired electron on iron. The second choice be<strong>in</strong>g correct is not surpris<strong>in</strong>g<br />
because s<strong>in</strong>glet oxygen <strong>and</strong> large separations <strong>of</strong> charge are both unfavorably high-<br />
energy states. Iron's shift to a higher oxidation state decreases the atom's size <strong>and</strong><br />
allows it <strong>in</strong>to the plane <strong>of</strong> the porphyr<strong>in</strong> r<strong>in</strong>g, pull<strong>in</strong>g on the coord<strong>in</strong>ated histid<strong>in</strong>e<br />
residue <strong>and</strong> <strong>in</strong>itiat<strong>in</strong>g the allosteric changes seen <strong>in</strong> the globul<strong>in</strong>s. The assignment <strong>of</strong><br />
oxidation state, however, is only a formalism so all three models may contribute to<br />
some small degree.<br />
Early postulates by bio<strong>in</strong>organic chemists claimed that possibility (1) (above) was<br />
correct <strong>and</strong> that iron should exist <strong>in</strong> oxidation state II (<strong>in</strong>deed iron oxidation state III as<br />
methemoglob<strong>in</strong>, when not accompanied by superoxide .O2 - to "hold" the oxidation<br />
electron, is <strong>in</strong>capable <strong>of</strong> b<strong>in</strong>d<strong>in</strong>g O2). The iron chemistry <strong>in</strong> this model was elegant, but<br />
the presence <strong>of</strong> s<strong>in</strong>glet oxygen was never expla<strong>in</strong>ed. It was argued that the b<strong>in</strong>d<strong>in</strong>g <strong>of</strong> an<br />
oxygen molecule placed high-sp<strong>in</strong> iron(II) <strong>in</strong> an octahedral field <strong>of</strong> strong-field lig<strong>and</strong>s;
this change <strong>in</strong> field would <strong>in</strong>crease the crystal field splitt<strong>in</strong>g energy, caus<strong>in</strong>g iron's<br />
electrons to pair <strong>in</strong>to the diamagnetic low-sp<strong>in</strong> configuration.<br />
B<strong>in</strong>d<strong>in</strong>g <strong>of</strong> lig<strong>and</strong>s<br />
B<strong>in</strong>d<strong>in</strong>g <strong>and</strong> release <strong>of</strong> lig<strong>and</strong>s <strong>in</strong>duces a conformational (structural) change <strong>in</strong><br />
hemoglob<strong>in</strong>. Here, the b<strong>in</strong>d<strong>in</strong>g <strong>and</strong> release <strong>of</strong> oxygen illustrates the structural<br />
differences between oxy- <strong>and</strong> deoxyhemoglob<strong>in</strong>, respectively. Only one <strong>of</strong> the four<br />
heme groups is shown.<br />
As discussed above, when oxygen b<strong>in</strong>ds to the iron center it causes contraction <strong>of</strong><br />
the iron atom, <strong>and</strong> causes it to move back <strong>in</strong>to the center <strong>of</strong> the porphyr<strong>in</strong> r<strong>in</strong>g plane<br />
(see mov<strong>in</strong>g diagram). At the same time, the porphyr<strong>in</strong> r<strong>in</strong>g plane itself is pushed away<br />
from the oxygen <strong>and</strong> toward the imidizole side cha<strong>in</strong> <strong>of</strong> the histid<strong>in</strong>e residue <strong>in</strong>teract<strong>in</strong>g<br />
at the other pole <strong>of</strong> the iron. The <strong>in</strong>teraction here forces the r<strong>in</strong>g plane sideways toward<br />
the outside <strong>of</strong> the tetramer, <strong>and</strong> also <strong>in</strong>duces a stra<strong>in</strong> on the prote<strong>in</strong> helix conta<strong>in</strong><strong>in</strong>g the<br />
histid<strong>in</strong>e, as it moves nearer the iron. This causes a tug on this peptide str<strong>and</strong> which<br />
tends to open up heme units <strong>in</strong> the rema<strong>in</strong>der <strong>of</strong> the molecule, so that there is more<br />
room for oxygen to b<strong>in</strong>d at their heme sites.<br />
In the tetrameric form <strong>of</strong> <strong>normal</strong> adult hemoglob<strong>in</strong>, the b<strong>in</strong>d<strong>in</strong>g <strong>of</strong> oxygen is thus a<br />
cooperative process. The b<strong>in</strong>d<strong>in</strong>g aff<strong>in</strong>ity <strong>of</strong> hemoglob<strong>in</strong> for oxygen is <strong>in</strong>creased by the<br />
oxygen saturation <strong>of</strong> the molecule, with the first oxygens bound <strong>in</strong>fluenc<strong>in</strong>g the shape
<strong>of</strong> the b<strong>in</strong>d<strong>in</strong>g sites for the next oxygens, <strong>in</strong> a way favorable for b<strong>in</strong>d<strong>in</strong>g. This positive<br />
cooperative b<strong>in</strong>d<strong>in</strong>g is achieved through steric conformational changes <strong>of</strong> the<br />
hemoglob<strong>in</strong> prote<strong>in</strong> complex as discussed above, i.e. when one subunit prote<strong>in</strong> <strong>in</strong><br />
hemoglob<strong>in</strong> becomes oxygenated, this <strong>in</strong>duces a conformational or structural change <strong>in</strong><br />
the whole complex, caus<strong>in</strong>g the other subunits to ga<strong>in</strong> an <strong>in</strong>creased aff<strong>in</strong>ity for oxygen.<br />
As a consequence, the oxygen b<strong>in</strong>d<strong>in</strong>g curve <strong>of</strong> hemoglob<strong>in</strong> is sigmoidal, or S-shaped,<br />
as opposed to the <strong>normal</strong> hyperbolic curve associated with noncooperative b<strong>in</strong>d<strong>in</strong>g.<br />
Hemoglob<strong>in</strong>'s oxygen-b<strong>in</strong>d<strong>in</strong>g capacity is decreased <strong>in</strong> the presence <strong>of</strong> carbon<br />
monoxide because both gases compete for the same b<strong>in</strong>d<strong>in</strong>g sites on hemoglob<strong>in</strong>,<br />
carbon monoxide b<strong>in</strong>d<strong>in</strong>g preferentially <strong>in</strong> place <strong>of</strong> oxygen. Carbon dioxide occupies a<br />
different b<strong>in</strong>d<strong>in</strong>g site on the hemoglob<strong>in</strong>. Through the enzyme carbonic anhydrase,<br />
carbon dioxide reacts with water to give carbonic acid, which decomposes <strong>in</strong>to<br />
bicarbonate <strong>and</strong> protons:<br />
CO2 + H2O → H2CO3 → HCO3 - + H +<br />
The sigmoidal shape <strong>of</strong> hemoglob<strong>in</strong>'s oxygen-dissociation curve results from<br />
cooperative b<strong>in</strong>d<strong>in</strong>g <strong>of</strong> oxygen to hemoglob<strong>in</strong>.<br />
Hence <strong>blood</strong> with high carbon dioxide levels is also lower <strong>in</strong> pH (more acidic).<br />
Hemoglob<strong>in</strong> can b<strong>in</strong>d protons <strong>and</strong> carbon dioxide which causes a conformational<br />
change <strong>in</strong> the prote<strong>in</strong> <strong>and</strong> facilitates the release <strong>of</strong> oxygen. Protons b<strong>in</strong>d at various
places along the prote<strong>in</strong>, <strong>and</strong> carbon dioxide b<strong>in</strong>ds at the alpha-am<strong>in</strong>o group form<strong>in</strong>g<br />
carbamate. Conversely, when the carbon dioxide levels <strong>in</strong> the <strong>blood</strong> decrease (i.e., <strong>in</strong><br />
the lung capillaries), carbon dioxide <strong>and</strong> protons are released from hemoglob<strong>in</strong>,<br />
<strong>in</strong>creas<strong>in</strong>g the oxygen aff<strong>in</strong>ity <strong>of</strong> the prote<strong>in</strong>. This control <strong>of</strong> hemoglob<strong>in</strong>'s aff<strong>in</strong>ity for<br />
oxygen by the b<strong>in</strong>d<strong>in</strong>g <strong>and</strong> release <strong>of</strong> carbon dioxide <strong>and</strong> acid, is known as the Bohr<br />
effect.<br />
The b<strong>in</strong>d<strong>in</strong>g <strong>of</strong> oxygen is affected by molecules such as carbon monoxide (CO)<br />
(for example from tobacco smok<strong>in</strong>g, cars <strong>and</strong> furnaces). CO competes with oxygen at<br />
the heme b<strong>in</strong>d<strong>in</strong>g site. Hemoglob<strong>in</strong> b<strong>in</strong>d<strong>in</strong>g aff<strong>in</strong>ity for CO is 200 times greater than its<br />
aff<strong>in</strong>ity for oxygen, mean<strong>in</strong>g that small amounts <strong>of</strong> CO dramatically reduces<br />
hemoglob<strong>in</strong>'s ability to transport oxygen. When hemoglob<strong>in</strong> comb<strong>in</strong>es with CO, it<br />
forms a very bright red compound called carboxyhemoglob<strong>in</strong>. When <strong>in</strong>spired air<br />
conta<strong>in</strong>s CO levels as low as 0.02%, headache <strong>and</strong> nausea occur; if the CO<br />
concentration is <strong>in</strong>creased to 0.1%, unconsciousness will follow. In heavy smokers, up<br />
to 20% <strong>of</strong> the oxygen-active sites can be blocked by CO.<br />
In similar fashion, hemoglob<strong>in</strong> also has competitive b<strong>in</strong>d<strong>in</strong>g aff<strong>in</strong>ity for cyanide<br />
(CN - ), sulfur monoxide (SO), nitrogen dioxide (NO2), <strong>and</strong> sulfide (S 2- ), <strong>in</strong>clud<strong>in</strong>g<br />
hydrogen sulfide (H2S). All <strong>of</strong> these b<strong>in</strong>d to iron <strong>in</strong> heme without chang<strong>in</strong>g its oxidation<br />
state, but they nevertheless <strong>in</strong>hibit oxygen-b<strong>in</strong>d<strong>in</strong>g, caus<strong>in</strong>g grave toxicity.<br />
The iron atom <strong>in</strong> the heme group must be <strong>in</strong> the Fe 2+ oxidation state to support<br />
oxygen <strong>and</strong> other gases' b<strong>in</strong>d<strong>in</strong>g <strong>and</strong> transport. Oxidation to Fe 3+ state converts<br />
hemoglob<strong>in</strong> <strong>in</strong>to hemiglob<strong>in</strong> or methemoglob<strong>in</strong> (pronounced "MET-hemoglob<strong>in</strong>"),<br />
which cannot b<strong>in</strong>d oxygen. Hemoglob<strong>in</strong> <strong>in</strong> <strong>normal</strong> red <strong>blood</strong> cells is protected by a<br />
reduction system to keep this from happen<strong>in</strong>g. Nitrogen dioxide <strong>and</strong> nitrous oxide are<br />
capable <strong>of</strong> convert<strong>in</strong>g a small fraction <strong>of</strong> hemoglob<strong>in</strong> to methemoglob<strong>in</strong>, however this<br />
is not usually <strong>of</strong> medical importance (nitrogen dioxide is poisonous by other<br />
mechanisms, <strong>and</strong> nitrous oxide is rout<strong>in</strong>ely used <strong>in</strong> surgical anesthesia <strong>in</strong> most people<br />
without undue methemoglob<strong>in</strong> buildup).
In people acclimated to high altitudes, the concentration <strong>of</strong> 2,3-<br />
bisphosphoglycerate (2,3-BPG) <strong>in</strong> the <strong>blood</strong> is <strong>in</strong>creased, which allows these<br />
<strong>in</strong>dividuals to deliver a larger amount <strong>of</strong> oxygen to tissues under conditions <strong>of</strong> lower<br />
oxygen tension. This phenomenon, where molecule Y affects the b<strong>in</strong>d<strong>in</strong>g <strong>of</strong> molecule X<br />
to a transport molecule Z, is called a heterotropic allosteric effect.<br />
A variant hemoglob<strong>in</strong>, called fetal hemoglob<strong>in</strong> (HbF, α2γ2), is found <strong>in</strong> the<br />
develop<strong>in</strong>g fetus, <strong>and</strong> b<strong>in</strong>ds oxygen with greater aff<strong>in</strong>ity than adult hemoglob<strong>in</strong>. This<br />
means that the oxygen b<strong>in</strong>d<strong>in</strong>g curve for fetal hemoglob<strong>in</strong> is left-shifted (i.e., a higher<br />
percentage <strong>of</strong> hemoglob<strong>in</strong> has oxygen bound to it at lower oxygen tension), <strong>in</strong><br />
comparison to that <strong>of</strong> adult hemoglob<strong>in</strong>. As a result, fetal <strong>blood</strong> <strong>in</strong> the placenta is able<br />
to take oxygen from maternal <strong>blood</strong>.<br />
Hemoglob<strong>in</strong> also carries nitric oxide <strong>in</strong> the glob<strong>in</strong> part <strong>of</strong> the molecule. This<br />
improves oxygen delivery <strong>in</strong> the periphery <strong>and</strong> contributes to the control <strong>of</strong> respiration.<br />
NO b<strong>in</strong>ds reversibly to a specific cyste<strong>in</strong> residue <strong>in</strong> glob<strong>in</strong>; the b<strong>in</strong>d<strong>in</strong>g depends on the<br />
state (R or T) <strong>of</strong> the hemoglob<strong>in</strong>. The result<strong>in</strong>g S-nitrosylated hemoglob<strong>in</strong> <strong>in</strong>fluences<br />
various NO-related activities such as the control <strong>of</strong> vascular resistance, <strong>blood</strong> pressure<br />
<strong>and</strong> respiration. NO is released not <strong>in</strong> the cytoplasm <strong>of</strong> erythrocytes but is transported<br />
by an anion exchanger called AE1 out <strong>of</strong> them. [7]<br />
Degradation <strong>of</strong> hemoglob<strong>in</strong> <strong>in</strong> vertebrate animals<br />
When red cells reach the end <strong>of</strong> their life due to ag<strong>in</strong>g or defects, they are broken<br />
down, the hemoglob<strong>in</strong> molecule is broken up <strong>and</strong> the iron gets recycled. When the<br />
porphyr<strong>in</strong> r<strong>in</strong>g is broken up, the fragments are <strong>normal</strong>ly secreted <strong>in</strong> the bile by the liver.<br />
This process also produces one molecule <strong>of</strong> carbon monoxide for every molecule <strong>of</strong><br />
heme degraded [4]; this is one <strong>of</strong> the few natural sources <strong>of</strong> carbon monoxide<br />
production <strong>in</strong> the human body, <strong>and</strong> is responsible for the <strong>normal</strong> <strong>blood</strong> levels <strong>of</strong> carbon<br />
monoxide even <strong>in</strong> people breath<strong>in</strong>g pure air. The other major f<strong>in</strong>al product <strong>of</strong> heme<br />
degradation is bilirub<strong>in</strong>. Increased levels <strong>of</strong> this chemical are detected <strong>in</strong> the <strong>blood</strong> if<br />
red cells are be<strong>in</strong>g destroyed more rapidly than usual. Improperly degraded hemoglob<strong>in</strong>
prote<strong>in</strong> or hemoglob<strong>in</strong> that has been released from the <strong>blood</strong> cells too rapidly can clog<br />
small <strong>blood</strong> vessels, especially the delicate <strong>blood</strong> filter<strong>in</strong>g vessels <strong>of</strong> the kidneys,<br />
caus<strong>in</strong>g kidney damage<br />
Role <strong>in</strong> disease<br />
Decrease <strong>of</strong> hemoglob<strong>in</strong>, with or without an absolute decrease <strong>of</strong> red <strong>blood</strong> cells,<br />
leads to symptoms <strong>of</strong> anemia. Anemia has many different causes, although iron<br />
deficiency <strong>and</strong> its resultant iron deficiency anemia are the most common causes <strong>in</strong> the<br />
Western world. As absence <strong>of</strong> iron decreases heme synthesis, red <strong>blood</strong> cells <strong>in</strong> iron<br />
deficiency anemia are hypochromic (lack<strong>in</strong>g the red hemoglob<strong>in</strong> pigment) <strong>and</strong><br />
microcytic (smaller than <strong>normal</strong>). Other anemias are rarer. In hemolysis (accelerated<br />
breakdown <strong>of</strong> red <strong>blood</strong> cells), associated jaundice is caused by the hemoglob<strong>in</strong><br />
metabolite bilirub<strong>in</strong>, <strong>and</strong> the circulat<strong>in</strong>g hemoglob<strong>in</strong> can cause renal failure.<br />
Some mutations <strong>in</strong> the glob<strong>in</strong> cha<strong>in</strong> are associated with the hemoglob<strong>in</strong>opathies,<br />
such as sickle-cell disease <strong>and</strong> thalassemia. Other mutations, as discussed at the<br />
beg<strong>in</strong>n<strong>in</strong>g <strong>of</strong> the article, are benign <strong>and</strong> are referred to merely as hemoglob<strong>in</strong> variants.<br />
There is a group <strong>of</strong> genetic disorders, known as the porphyrias that are<br />
characterized by errors <strong>in</strong> metabolic pathways <strong>of</strong> heme synthesis. K<strong>in</strong>g George III <strong>of</strong> the<br />
United K<strong>in</strong>gdom was probably the most famous porphyria sufferer.<br />
To a small extent, hemoglob<strong>in</strong> A slowly comb<strong>in</strong>es with glucose at a certa<strong>in</strong><br />
location <strong>in</strong> the molecule. The result<strong>in</strong>g molecule is <strong>of</strong>ten referred to as Hb A1c. As the<br />
concentration <strong>of</strong> glucose <strong>in</strong> the <strong>blood</strong> <strong>in</strong>creases, the percentage <strong>of</strong> Hb A that turns <strong>in</strong>to<br />
Hb A1c <strong>in</strong>creases. In diabetics whose glucose usually runs high, the percent Hb A1c also<br />
runs high. Because <strong>of</strong> the slow rate <strong>of</strong> Hb A comb<strong>in</strong>ation with glucose, the Hb A1c<br />
percentage is representative <strong>of</strong> glucose level <strong>in</strong> the <strong>blood</strong> averaged over a longer time<br />
(the half-life <strong>of</strong> red <strong>blood</strong> cells, which is typically 50-55 days).
Diagnostic use<br />
Hemoglob<strong>in</strong> levels are amongst the most commonly performed <strong>blood</strong> tests, usually<br />
as part <strong>of</strong> a full <strong>blood</strong> count or complete <strong>blood</strong> count. Results are reported <strong>in</strong> g/L, g/dL<br />
or mol/L. For conversion, 1 g/dL is 0.621 mmol/L. If the total hemoglob<strong>in</strong><br />
concentration <strong>in</strong> the <strong>blood</strong> falls below a set po<strong>in</strong>t, this is called anemia. Normal values<br />
for hemoglob<strong>in</strong> levels are:<br />
15.1 g/dl<br />
g/dl<br />
g/dl<br />
11 to 12 g/dl [5]<br />
Women: 12.1 to<br />
Men: 13.8 to 17.2<br />
Children: 11 to 16<br />
Pregnant women:<br />
Anemias are further subclassified by the size <strong>of</strong> the red <strong>blood</strong> cells, which are the<br />
cells which conta<strong>in</strong> hemoglob<strong>in</strong> <strong>in</strong> vertebrates. They can be classified as microcytic<br />
(small sized red <strong>blood</strong> cells), normocytic (<strong>normal</strong> sized red <strong>blood</strong> cells), or macrocytic<br />
(large sized red <strong>blood</strong> cells). The hemaglob<strong>in</strong> is the typical test used for <strong>blood</strong> donation.<br />
A comparison with the hematocrit can be made by multiply<strong>in</strong>g the hemaglob<strong>in</strong> by three.<br />
For example, if the hemaglob<strong>in</strong> is measured at 17, that compares with a hematocrit <strong>of</strong><br />
.51.[6]<br />
Glucose levels <strong>in</strong> <strong>blood</strong> can vary widely each hour, so one or only a few samples<br />
from a patient analyzed for glucose may not be representative <strong>of</strong> glucose control <strong>in</strong> the<br />
long run. For this reason a <strong>blood</strong> sample may be analyzed for Hb A1c level, which is<br />
more representative <strong>of</strong> glucose control averaged over a longer time period (determ<strong>in</strong>ed<br />
by the half-life <strong>of</strong> the <strong>in</strong>dividual's red <strong>blood</strong> cells, which is typically 50-55 days).<br />
People whose Hb A1c runs 6.0% or less show good longer-term glucose control. Hb A1c
values which are more than 7.0% are elevated. This<br />
test is especially useful for diabetics. [8]<br />
Lymphocytes<br />
There are several k<strong>in</strong>ds <strong>of</strong> lymphocytes<br />
(although they all look alike under the microscope),<br />
each with different functions to perform . The most<br />
common types <strong>of</strong> lymphocytes are<br />
B lymphocytes ("B cells"). These are responsible for mak<strong>in</strong>g antibodies.<br />
T lymphocytes ("T cells"). There are several subsets <strong>of</strong> these:<br />
o <strong>in</strong>flammatory T cells that recruit macrophages <strong>and</strong><br />
neutrophils to the site <strong>of</strong> <strong>in</strong>fection or other tissue damage<br />
o cytotoxic T lymphocytes (CTLs) that kill virus-<strong>in</strong>fected <strong>and</strong>,<br />
perhaps, tumor cells<br />
cells<br />
o helper T cells that enhance the production <strong>of</strong> antibodies by B<br />
Although bone marrow is the ultimate source <strong>of</strong> lymphocytes, the lymphocytes<br />
that will become T cells migrate from the bone marrow to the thymus where they<br />
mature. Both B cells <strong>and</strong> T cells also take up residence <strong>in</strong> lymph nodes, the spleen <strong>and</strong><br />
other tissues where they<br />
Monocytes<br />
encounter antigens;<br />
cont<strong>in</strong>ue to divide by mitosis;<br />
mature <strong>in</strong>to fully functional cells.<br />
Monocytes leave the <strong>blood</strong> <strong>and</strong> become macrophages <strong>and</strong> dendritic cells.
This scann<strong>in</strong>g electron micrograph (courtesy <strong>of</strong> Drs. Jan M. Orenste<strong>in</strong> <strong>and</strong> Emma<br />
Shelton) shows a s<strong>in</strong>gle macrophage surrounded by several lymphocytes.<br />
Macrophages are large, phagocytic cells that engulf<br />
Platelets<br />
foreign material (antigens) that enter the body<br />
dead <strong>and</strong> dy<strong>in</strong>g cells <strong>of</strong> the body.<br />
Platelets are cell fragments produced from megakaryocytes.<br />
Blood <strong>normal</strong>ly conta<strong>in</strong>s 150,000–350,000 per microliter (µl) or cubic millimeter<br />
(mm 3 ). This number is <strong>normal</strong>ly ma<strong>in</strong>ta<strong>in</strong>ed by a homeostatic (negative-feedback)<br />
mechanism .<br />
If this value should drop much below 50,000/µl, there is a danger <strong>of</strong> uncontrolled<br />
bleed<strong>in</strong>g because <strong>of</strong> the essential role that platelets have <strong>in</strong> <strong>blood</strong> clott<strong>in</strong>g.<br />
Some causes:<br />
certa<strong>in</strong> drugs <strong>and</strong> herbal remedies;<br />
autoimmunity.<br />
When <strong>blood</strong> vessels are cut or damaged, the loss <strong>of</strong> <strong>blood</strong> from the system must be<br />
stopped before shock <strong>and</strong> possible death occur. This is accomplished by solidification<br />
<strong>of</strong> the <strong>blood</strong>, a process called coagulation or clott<strong>in</strong>g.<br />
A <strong>blood</strong> clot consists <strong>of</strong><br />
Plasma<br />
a plug <strong>of</strong> platelets enmeshed <strong>in</strong> a<br />
network <strong>of</strong> <strong>in</strong>soluble fibr<strong>in</strong> molecules.<br />
Plasma is the straw-colored liquid <strong>in</strong> which the <strong>blood</strong> cells are suspended.
sugar)<br />
Composition <strong>of</strong> <strong>blood</strong><br />
plasma<br />
Component<br />
Water<br />
Prote<strong>in</strong>s<br />
Pe<br />
rcent<br />
2<br />
8<br />
~9<br />
6–<br />
Salts 0.8<br />
Lipids 0.6<br />
Glucose (<strong>blood</strong><br />
0.1<br />
Plasma transports materials needed by cells <strong>and</strong> materials that must be removed<br />
from cells:<br />
various ions (Na + , Ca 2+ , HCO3 − , etc.<br />
glucose <strong>and</strong> traces <strong>of</strong> other sugars<br />
am<strong>in</strong>o acids<br />
other organic acids<br />
cholesterol <strong>and</strong> other lipids<br />
hormones<br />
urea <strong>and</strong> other wastes<br />
Most <strong>of</strong> these materials are <strong>in</strong> transit from a place where they are added to the<br />
<strong>blood</strong> (a "source")<br />
exchange organs like the <strong>in</strong>test<strong>in</strong>e<br />
depots <strong>of</strong> materials like the liver<br />
to places ("s<strong>in</strong>ks") where they will be removed from the <strong>blood</strong>.
every cell<br />
exchange organs like the kidney, <strong>and</strong> sk<strong>in</strong>.<br />
Serum Prote<strong>in</strong>s<br />
Prote<strong>in</strong>s make up 6–8% <strong>of</strong> the <strong>blood</strong>. They are<br />
about equally divided between serum album<strong>in</strong> <strong>and</strong> a great<br />
variety <strong>of</strong> serum globul<strong>in</strong>s.<br />
After <strong>blood</strong> is withdrawn from a ve<strong>in</strong> <strong>and</strong><br />
allowed to clot, the clot slowly shr<strong>in</strong>ks. As it does so, a clear fluid called serum is<br />
squeezed out. Thus:<br />
Serum is <strong>blood</strong> plasma without fibr<strong>in</strong>ogen <strong>and</strong> other clott<strong>in</strong>g factors.<br />
The serum prote<strong>in</strong>s can be separated by electrophoresis.<br />
A drop <strong>of</strong> serum is applied <strong>in</strong> a b<strong>and</strong> to a th<strong>in</strong> sheet <strong>of</strong> support<strong>in</strong>g material,<br />
like paper, that has been soaked <strong>in</strong> a slightly-alkal<strong>in</strong>e salt solution.<br />
At pH 8.6, which is commonly used, all the prote<strong>in</strong>s are negatively<br />
charged, but some more strongly than others.<br />
A direct current can flow through the paper because <strong>of</strong> the conductivity <strong>of</strong><br />
the buffer with which it is moistened.<br />
electrode.<br />
As the current flows, the serum prote<strong>in</strong>s move toward the positive<br />
The stronger the negative charge on a prote<strong>in</strong>, the faster it migrates.<br />
After a time (typically 20 m<strong>in</strong>), the current is turned <strong>of</strong>f <strong>and</strong> the prote<strong>in</strong>s<br />
sta<strong>in</strong>ed to make them visible (most are otherwise colorless).<br />
The separated prote<strong>in</strong>s appear as dist<strong>in</strong>ct b<strong>and</strong>s.<br />
The most prom<strong>in</strong>ent <strong>of</strong> these <strong>and</strong> the one that moves closest to the positive<br />
electrode is serum album<strong>in</strong>.
Serum album<strong>in</strong><br />
o is made <strong>in</strong> the liver<br />
o b<strong>in</strong>ds many small molecules for transport through the <strong>blood</strong><br />
o helps ma<strong>in</strong>ta<strong>in</strong> the osmotic pressure <strong>of</strong> the <strong>blood</strong><br />
The other prote<strong>in</strong>s are the various serum globul<strong>in</strong>s.<br />
They migrate <strong>in</strong> the order<br />
o alpha globul<strong>in</strong>s (e.g., the prote<strong>in</strong>s that transport thyrox<strong>in</strong>e <strong>and</strong><br />
ret<strong>in</strong>ol [vitam<strong>in</strong> A])<br />
o beta globul<strong>in</strong>s (e.g., the iron-transport<strong>in</strong>g prote<strong>in</strong> transferr<strong>in</strong>)<br />
o gamma globul<strong>in</strong>s.<br />
Gamma globul<strong>in</strong>s are the least negatively-charged serum<br />
prote<strong>in</strong>s. (They are so weakly charged, <strong>in</strong> fact, that some are swept <strong>in</strong> the<br />
flow <strong>of</strong> buffer back toward the negative electrode.)<br />
Most antibodies are gamma globul<strong>in</strong>s.<br />
Therefore gamma globul<strong>in</strong>s become more abundant follow<strong>in</strong>g<br />
<strong>in</strong>fections or immunizations.<br />
Album<strong>in</strong>s – multidispersed fraction <strong>of</strong> <strong>blood</strong> plasma which are characterized by<br />
the high electrophoretic mobility <strong>and</strong> mild dissolubility <strong>in</strong> water <strong>and</strong> sal<strong>in</strong>e solutions.<br />
Molecular weight <strong>of</strong> album<strong>in</strong>s is about 60000. Due to high hydrophilic properties<br />
album<strong>in</strong>s b<strong>in</strong>d a significant amount <strong>of</strong> water, <strong>and</strong> the volume <strong>of</strong> their molecule under<br />
hydratation is doubled. Hydrative layer formed around the serum album<strong>in</strong>s provides to<br />
70-80 % <strong>of</strong> oncotic pressure <strong>of</strong> <strong>blood</strong> plasma prote<strong>in</strong>s, that can be applied <strong>in</strong> cl<strong>in</strong>ical<br />
practice at album<strong>in</strong>s transfusion to patients with tissue edemas. The decreas<strong>in</strong>g <strong>of</strong><br />
album<strong>in</strong>s concentration <strong>in</strong> <strong>blood</strong> plasma, for example under disturbance <strong>of</strong> their<br />
synthesis <strong>in</strong> hepatocytes at liver failure, can cause the water transition from a vessels<br />
<strong>in</strong>to the tissues <strong>and</strong> development <strong>of</strong> oncotic edemas.<br />
Album<strong>in</strong>s execute also important physiological function as transporters <strong>of</strong> a lot <strong>of</strong><br />
metabolites <strong>and</strong> diverse low molecular weight structures. The molecules <strong>of</strong> album<strong>in</strong>s
have several sites with centers <strong>of</strong> l<strong>in</strong>kage for molecules <strong>of</strong> organic lig<strong>and</strong>s, which are<br />
affixed by the electrostatic <strong>and</strong> hydrophobic bonds. Serum album<strong>in</strong>s can affix <strong>and</strong><br />
convey fatty acids, cholesterol, cholic pigments (bilirub<strong>in</strong> <strong>and</strong> that similar), vitam<strong>in</strong>s,<br />
hormones, some am<strong>in</strong>o acids, tox<strong>in</strong>s <strong>and</strong> medic<strong>in</strong>es.<br />
Album<strong>in</strong>s also execute the buffer function. Due to the availability <strong>in</strong> their structure<br />
am<strong>in</strong>o <strong>and</strong> carboxylic groups album<strong>in</strong>s can react both as acids <strong>and</strong> as alkal<strong>in</strong>e.<br />
Album<strong>in</strong>s can bound different tox<strong>in</strong>s <strong>in</strong> <strong>blood</strong> plasma (bilirub<strong>in</strong>, foreign<br />
substances et c.). This is the des<strong>in</strong>toxicative function <strong>of</strong> album<strong>in</strong>s.<br />
Album<strong>in</strong>s also play role <strong>of</strong> am<strong>in</strong>o acids depot <strong>in</strong> the organism. They can supply<br />
am<strong>in</strong>o acids for the build<strong>in</strong>g <strong>of</strong> another prote<strong>in</strong>s, for example enzymes.<br />
Globul<strong>in</strong>s - heterogeneous fraction <strong>of</strong> <strong>blood</strong> prote<strong>in</strong>s which execute transport ( 1-<br />
globul<strong>in</strong>s – transport <strong>of</strong> lipids, thyrox<strong>in</strong>, corticosteroid hormones; 2-globul<strong>in</strong>s -<br />
transport <strong>of</strong> lipids, copper ions; -globul<strong>in</strong>s - transport <strong>of</strong> lipids, iron) <strong>and</strong> protective<br />
(participation <strong>of</strong> -globul<strong>in</strong>s <strong>in</strong> immune reactions as antitox<strong>in</strong>s; -globul<strong>in</strong>s as<br />
immunoglobul<strong>in</strong>s) functions. They also support the <strong>blood</strong> oncotic pressure <strong>and</strong> acid-<br />
alkal<strong>in</strong>e balance, provide am<strong>in</strong>o acids for the organism requirements. The molecular<br />
weight <strong>of</strong> globul<strong>in</strong>s is approximately 150000-300000.<br />
The globul<strong>in</strong> level <strong>in</strong> <strong>blood</strong> plasma is 20-40 g/l. A ratio between concentrations <strong>of</strong><br />
album<strong>in</strong>s <strong>and</strong> globul<strong>in</strong>s (so called ―prote<strong>in</strong> coefficient‖) <strong>in</strong> <strong>blood</strong> plasma is <strong>of</strong>ten<br />
determ<strong>in</strong>ed <strong>in</strong> cl<strong>in</strong>ical practice. In healthy people this coefficient is 1,5-2,0.<br />
Fibr<strong>in</strong>ogen – important prote<strong>in</strong> <strong>of</strong> <strong>blood</strong> plasma, precursor <strong>of</strong> fibr<strong>in</strong>, the structural<br />
element <strong>of</strong> <strong>blood</strong> clots. Fibr<strong>in</strong>ogen participates <strong>in</strong> <strong>blood</strong> clott<strong>in</strong>g <strong>and</strong> thus prevents the<br />
loss <strong>of</strong> <strong>blood</strong> from the vascular system <strong>of</strong> vertebrates. The approximate molecular<br />
weight <strong>of</strong> fibr<strong>in</strong>ogen is 340000. It is the complex prote<strong>in</strong>, it conta<strong>in</strong>s the carbohydrate<br />
as prosthetic group. The content <strong>of</strong> fir<strong>in</strong>ogen <strong>in</strong> <strong>blood</strong> is 3-4 g/l.<br />
Subfractions <strong>of</strong> 1, 2, <strong>and</strong> globul<strong>in</strong>s, their structure <strong>and</strong> functions.<br />
Immunoglobul<strong>in</strong>s (Ig A, Ig G, Ig E, Ig M) - prote<strong>in</strong>s <strong>of</strong> -globul<strong>in</strong> fraction <strong>of</strong><br />
<strong>blood</strong> plasma execut<strong>in</strong>g the functions <strong>of</strong> antibodies which are the ma<strong>in</strong> effectors <strong>of</strong><br />
humoral immunity. They appear <strong>in</strong> the <strong>blood</strong> serum <strong>and</strong> certa<strong>in</strong> cells <strong>of</strong> a vertebrate <strong>in</strong>
esponse to the <strong>in</strong>troduction <strong>of</strong> a prote<strong>in</strong> or some other macromolecule foreign to that<br />
species.<br />
Immunoglobul<strong>in</strong> molecules have b<strong>in</strong>d<strong>in</strong>d sites that are specific for <strong>and</strong><br />
complementary to the structural features <strong>of</strong> the antigen that <strong>in</strong>duced their formation.<br />
Antibodies are highly specific for the foreign prote<strong>in</strong>s that evoke their formation.<br />
Molecules <strong>of</strong> immunoglobul<strong>in</strong>s are glycoprote<strong>in</strong>s. The prote<strong>in</strong> part <strong>of</strong><br />
immunoglobul<strong>in</strong>s conta<strong>in</strong> four polipeptide cha<strong>in</strong>s: two heavy H-cha<strong>in</strong>s <strong>and</strong> two light L-<br />
cha<strong>in</strong>s.<br />
C-reactive prote<strong>in</strong> ( -fraction). This prote<strong>in</strong> received the title ow<strong>in</strong>g to its capacity<br />
to react with C-polysaccharide <strong>of</strong> a pneumococcus form<strong>in</strong>g precipitates. Accord<strong>in</strong>g to<br />
its chemical nature C-reactive prote<strong>in</strong> is glycoprote<strong>in</strong>.<br />
In <strong>blood</strong> plasma <strong>of</strong> healthy people the C-reactive prote<strong>in</strong> is absent but it occurs at<br />
<strong>pathological</strong> states accompanied by an <strong>in</strong>flammation <strong>and</strong> necrosis <strong>of</strong> tissues. The<br />
availability <strong>of</strong> C-reactive prote<strong>in</strong> is characteristic for the acute period <strong>of</strong> diseases –<br />
―prote<strong>in</strong> <strong>of</strong> an acute phase‖. The determ<strong>in</strong>ation <strong>of</strong> C-reactive prote<strong>in</strong> has diagnostic<br />
value <strong>in</strong> an acute phase <strong>of</strong> rheumatic disease, at a myocardial <strong>in</strong>farction, pneumococcal,<br />
streptococcal, staphylococcal <strong>in</strong>fections.<br />
Crioglobul<strong>in</strong> - the prote<strong>in</strong> <strong>of</strong> the -globul<strong>in</strong> fraction. Like to the C-reactive prote<strong>in</strong><br />
crioglobul<strong>in</strong> absent <strong>in</strong> <strong>blood</strong> plasma <strong>of</strong> the healthy people <strong>and</strong> occurs at leukoses,<br />
rheumatic disease, liver cirrhosis, nephroses. The characteristic physico-chemical<br />
feature <strong>of</strong> crioglobul<strong>in</strong> is its dissolubility at st<strong>and</strong>ard body temperature (37 o C) <strong>and</strong><br />
capacity to form the sediment at cool<strong>in</strong>g <strong>of</strong> a <strong>blood</strong> plasma up to 4 o C.<br />
2-macroglobul<strong>in</strong> - prote<strong>in</strong> <strong>of</strong> 2-globul<strong>in</strong> fraction, universal serum prote<strong>in</strong>ase<br />
<strong>in</strong>hibitor. Its contents (2,5 g/l) <strong>in</strong> <strong>blood</strong> plasma is highest compar<strong>in</strong>g to another<br />
prote<strong>in</strong>ase <strong>in</strong>hibitors.<br />
The biological role <strong>of</strong> 2-macroglobul<strong>in</strong> consists <strong>in</strong> regulation <strong>of</strong> the tissue<br />
proteolysis systems which are very important <strong>in</strong> such physiological <strong>and</strong> <strong>pathological</strong><br />
processes as <strong>blood</strong> clott<strong>in</strong>g, fibr<strong>in</strong>olysis, processes <strong>of</strong> immunodefence, functionality <strong>of</strong> a<br />
complement system, <strong>in</strong>flammation, regulation <strong>of</strong> vascular tone (k<strong>in</strong><strong>in</strong>e <strong>and</strong> ren<strong>in</strong>-<br />
angiothens<strong>in</strong>e system).
1-antitryps<strong>in</strong> ( 1-globul<strong>in</strong>) – glycoprote<strong>in</strong> with a molecular weight 55 kDa. Its<br />
concentration <strong>in</strong> <strong>blood</strong> plasma is 2-3 г/л. The ma<strong>in</strong> biological property <strong>of</strong> this <strong>in</strong>hibitor<br />
is its capacity to form complexes with prote<strong>in</strong>ases oppress<strong>in</strong>g proteolitic activity <strong>of</strong><br />
such enzymes as tryps<strong>in</strong>, chemotryps<strong>in</strong>, plasm<strong>in</strong>, tromb<strong>in</strong>. The content <strong>of</strong> 1-antitryps<strong>in</strong><br />
is markedly <strong>in</strong>creased <strong>in</strong> <strong>in</strong>flammatory processes. The <strong>in</strong>hibitory activity <strong>of</strong> 1-<br />
antitryps<strong>in</strong> is very important <strong>in</strong> pancreas necrosis <strong>and</strong> acute pancreatitis because <strong>in</strong> these<br />
conditions the prote<strong>in</strong>ase level <strong>in</strong> <strong>blood</strong> <strong>and</strong> tissues is sharply <strong>in</strong>creased. The congenital<br />
deficiency <strong>of</strong> 1-antitryps<strong>in</strong> results <strong>in</strong> the lung emphysema.<br />
Fibronect<strong>in</strong> – glycoprote<strong>in</strong> <strong>of</strong> <strong>blood</strong> plasma that is synthesized <strong>and</strong> secreted <strong>in</strong><br />
<strong>in</strong>tercellular space by different cells. Fibronect<strong>in</strong> present on a surface <strong>of</strong> cells, on the<br />
basal membranes, <strong>in</strong> connective tissue <strong>and</strong> <strong>in</strong> <strong>blood</strong>. Fibronect<strong>in</strong> has properties <strong>of</strong> a<br />
«stick<strong>in</strong>g» prote<strong>in</strong> <strong>and</strong> contacts with the carbohydrate groups <strong>of</strong> gangliosides on a<br />
surface <strong>of</strong> plasma membranes execut<strong>in</strong>g the <strong>in</strong>tegrative function <strong>in</strong> <strong>in</strong>tercellular<br />
<strong>in</strong>terplay. Fibronect<strong>in</strong> also plays important role <strong>in</strong> the formation <strong>of</strong> the pericellular<br />
matrix.<br />
Haptoglob<strong>in</strong> - prote<strong>in</strong> <strong>of</strong> 2-globul<strong>in</strong> fraction <strong>of</strong> <strong>blood</strong> plasma. Haptoglob<strong>in</strong> has<br />
capacity to b<strong>in</strong>d a free haemoglob<strong>in</strong> form<strong>in</strong>g a complex that refer to -globul<strong>in</strong>s<br />
electrophoretic fraction. Normal concentration <strong>in</strong> <strong>blood</strong> plasma - 0,10-0,35 g/l.<br />
Haptoglob<strong>in</strong>-hemoglob<strong>in</strong> complexes are absorbed by the cells <strong>of</strong> reticulo-<br />
endothelial system, <strong>in</strong> particular <strong>in</strong> a liver, <strong>and</strong> oxidized to cholic pigments. Such<br />
haptoglob<strong>in</strong> function promotes the preservation <strong>of</strong> iron ions <strong>in</strong> an organism under<br />
conditions <strong>of</strong> a physiological <strong>and</strong> <strong>pathological</strong> erythrocytolysis.<br />
Transferr<strong>in</strong> - glycoprote<strong>in</strong> belong<strong>in</strong>g to the -globul<strong>in</strong> fraction. It b<strong>in</strong>ds <strong>in</strong> a <strong>blood</strong><br />
plasma iron ions (Fe 3+ ). The prote<strong>in</strong> has on the surface two centers <strong>of</strong> l<strong>in</strong>kage <strong>of</strong> iron.<br />
Transferr<strong>in</strong> is a transport form <strong>of</strong> iron deliver<strong>in</strong>g its to places <strong>of</strong> accumulation <strong>and</strong><br />
usage.<br />
Ceruloplasm<strong>in</strong> - glycoprote<strong>in</strong> <strong>of</strong> the 2-globul<strong>in</strong> fraction. It can b<strong>in</strong>d the copper<br />
ions <strong>in</strong> <strong>blood</strong> plasma. Up to 3 % <strong>of</strong> all copper contents <strong>in</strong> an organism <strong>and</strong> more than 90<br />
% copper contents <strong>in</strong> plasma is <strong>in</strong>cluded <strong>in</strong> ceruloplasm<strong>in</strong>. Ceruloplasm<strong>in</strong> has properties<br />
<strong>of</strong> ferroxidase oxidiz<strong>in</strong>g the iron ions. The decrease <strong>of</strong> ceruloplasm<strong>in</strong> <strong>in</strong> organism
(Wilson disease) results <strong>in</strong> exit <strong>of</strong> copper ions from vessels <strong>and</strong> its accumulation <strong>in</strong> the<br />
connective tissue that shows by <strong>pathological</strong> changes <strong>in</strong> a liver, ma<strong>in</strong> bra<strong>in</strong>, cornea.<br />
The place <strong>of</strong> synthesis <strong>of</strong> each fraction <strong>and</strong> subfruction <strong>of</strong> <strong>blood</strong> plasma prote<strong>in</strong>s.<br />
Album<strong>in</strong>s, 1-globul<strong>in</strong>s, fibr<strong>in</strong>ogen are fully synthesized <strong>in</strong> hepatocytes.<br />
Immunoglobul<strong>in</strong>s are produced by plasmocytes (immune cells). In liver cryoglobul<strong>in</strong>s<br />
<strong>and</strong> some other -globul<strong>in</strong>s are produced too. 2-globul<strong>in</strong>s <strong>and</strong> -globul<strong>in</strong>s are partly<br />
synthesized <strong>in</strong> liver <strong>and</strong> partly <strong>in</strong> reticuloendothelial cells.<br />
Causes <strong>and</strong> consequences <strong>of</strong> prote<strong>in</strong> content changes <strong>in</strong> <strong>blood</strong> plasma.<br />
Hypoprote<strong>in</strong>emia - decrease <strong>of</strong> the total contents <strong>of</strong> prote<strong>in</strong>s <strong>in</strong> <strong>blood</strong> plasma. This<br />
state occurs <strong>in</strong> old people as well as <strong>in</strong> <strong>pathological</strong> states accompany<strong>in</strong>g with the<br />
oppress<strong>in</strong>g <strong>of</strong> prote<strong>in</strong> synthesis (liver diseases) <strong>and</strong> activation <strong>of</strong> decomposition <strong>of</strong><br />
tissue prote<strong>in</strong>s (starvation, hard <strong>in</strong>fectious diseases, state after hard trauma <strong>and</strong><br />
operations, cancer). Hypoprote<strong>in</strong>emia (hypoalbum<strong>in</strong>emia) also occurs <strong>in</strong> kidney<br />
diseases, when the <strong>in</strong>creased excretion <strong>of</strong> prote<strong>in</strong>s via the ur<strong>in</strong>e takes place.<br />
Hyperprote<strong>in</strong>emia - <strong>in</strong>crease <strong>of</strong> the total contents <strong>of</strong> prote<strong>in</strong>s <strong>in</strong> <strong>blood</strong> plasma.<br />
There are two types <strong>of</strong> hyperprote<strong>in</strong>emia - absolute <strong>and</strong> relative.<br />
Absolute hyperprote<strong>in</strong>emia – accumulation <strong>of</strong> the prote<strong>in</strong>s <strong>in</strong> <strong>blood</strong>. It occurs <strong>in</strong><br />
<strong>in</strong>fection <strong>and</strong> <strong>in</strong>flammatory diseases (hyperproduction <strong>of</strong> immunoglobul<strong>in</strong>s), rheumatic<br />
diseases (hyperproduction <strong>of</strong> C-reactive prote<strong>in</strong>), some malignant tumors (myeloma)<br />
<strong>and</strong> others.<br />
Relative hyperprote<strong>in</strong>emia – the <strong>in</strong>crease <strong>of</strong> the prote<strong>in</strong> concentration but not the<br />
absolute amount <strong>of</strong> prote<strong>in</strong>s. It occurs when organism loses water (diarrhea, vomit<strong>in</strong>g,<br />
fever, <strong>in</strong>tensive physical activity etc.).<br />
The pr<strong>in</strong>ciple <strong>of</strong> the measurement <strong>of</strong> prote<strong>in</strong> fractions by electrophoresis method.<br />
Electrophoresis is the separation <strong>of</strong> prote<strong>in</strong>s on the basis <strong>of</strong> their electric charge. It<br />
depends ultimately on their base-acid properties, which are largely determ<strong>in</strong>ed by the<br />
number <strong>and</strong> types <strong>of</strong> ionizable R groups <strong>in</strong> their polipeptide cha<strong>in</strong>s. S<strong>in</strong>ce prote<strong>in</strong>s<br />
differ <strong>in</strong> am<strong>in</strong>o acid composition <strong>and</strong> sequence, each prote<strong>in</strong> has dist<strong>in</strong>ctive acid-base<br />
properties. There are a number <strong>of</strong> different forms <strong>of</strong> electr<strong>of</strong>oresis useful for analyz<strong>in</strong>g<br />
<strong>and</strong> separat<strong>in</strong>g mixtures <strong>of</strong> prote<strong>in</strong>s
If a precursor <strong>of</strong> an antibody-secret<strong>in</strong>g<br />
cell becomes cancerous, it divides<br />
uncontrollably to generate a clone <strong>of</strong><br />
plasma cells secret<strong>in</strong>g a s<strong>in</strong>gle k<strong>in</strong>d <strong>of</strong> antibody molecule. The image<br />
(courtesy <strong>of</strong> Beckman Instruments, Inc.) shows — from left to right —<br />
the electrophoretic separation <strong>of</strong>:<br />
globul<strong>in</strong>s;<br />
1. <strong>normal</strong> human serum with its diffuse b<strong>and</strong> <strong>of</strong> gamma<br />
2. serum from a patient with multiple myeloma produc<strong>in</strong>g an<br />
IgG myeloma prote<strong>in</strong>;<br />
3. serum from a patient with Waldenström's macroglobul<strong>in</strong>emia<br />
where the cancerous clone secretes an IgM antibody;<br />
4. serum with an IgA myeloma prote<strong>in</strong>.<br />
Gamma globul<strong>in</strong>s can be harvested from donated <strong>blood</strong> (usually<br />
pooled from several thous<strong>and</strong> donors) <strong>and</strong> <strong>in</strong>jected <strong>in</strong>to persons exposed to<br />
certa<strong>in</strong> diseases such as chicken pox <strong>and</strong> hepatitis. Because such preparations <strong>of</strong><br />
immune globul<strong>in</strong> conta<strong>in</strong> antibodies aga<strong>in</strong>st most common <strong>in</strong>fectious diseases,<br />
the patient ga<strong>in</strong>s temporary protection aga<strong>in</strong>st the disease.<br />
Serum Lipids<br />
Because <strong>of</strong> their relationship to cardiovascular disease, the analysis <strong>of</strong> serum lipids<br />
has become an important health measure.<br />
The table shows the range <strong>of</strong> typical values as well as the values above (or below)<br />
which the subject may be at <strong>in</strong>creased risk <strong>of</strong> develop<strong>in</strong>g atherosclerosis.<br />
LIPID<br />
Typical values<br />
(mg/dl)<br />
Desirable<br />
(mg/dl)
(total)<br />
Cholesterol<br />
LDL<br />
cholesterol<br />
HDL<br />
cholesterol<br />
s<br />
Triglyceride<br />
170–210
agar gel.<br />
B. Prote<strong>in</strong> fractions which are received with the help <strong>of</strong> imunoelectropheresis on<br />
Prote<strong>in</strong> Concentration<br />
Acidic α1<br />
glycoproteid<br />
(Ig)<br />
Fractions Concentration Relative contents<br />
Album<strong>in</strong> 38,0 - 50,0 g/l 0,50 - 0,60<br />
α1 globul<strong>in</strong>s 1,4 – 3,0 g/l 0,01 - 0,05<br />
α2 globul<strong>in</strong>s 5,6 – 9,1 g/l 0,07 - 0,13<br />
β- globul<strong>in</strong>s 5,4 – 9,1 g/l 0,09 – 0,15<br />
γ globul<strong>in</strong>s 9,1 – 14,7 g/l 0,14 – 0,22<br />
Total prote<strong>in</strong> 65,0 – 85, 0 g/l 1,00<br />
α1Antitrypsyn<br />
Ceruloplasm<strong>in</strong><br />
Cu 2+<br />
Haptoglob<strong>in</strong>e<br />
α2 - Macroglobul<strong>in</strong><br />
Transpheryn<br />
Fe 3+<br />
Fibr<strong>in</strong>ogen<br />
Immunoglobul<strong>in</strong>s<br />
IgG<br />
IgA<br />
IgM<br />
IgD<br />
IgE<br />
16,0-31,0<br />
mkmmol/l<br />
11,0-27,0<br />
mkmmol/l<br />
0,20 – 0,40 g/l<br />
2,00-4,00 g/l<br />
0,15-0,60 g/l<br />
1,00-4,00 g/l<br />
2,50-3,50 g/l<br />
2,50-4,10 g/l<br />
2,00-4,00 g/l<br />
8,00-18,00 g/l<br />
1,00-4,00 g/l<br />
0,60-2,80 g/l<br />
0,00-0,15 g/l<br />
Till 5x10 -4
Residual nitrogen, its <strong>components</strong>, ways <strong>of</strong> their formation, <strong>blood</strong><br />
content<br />
The state <strong>of</strong> prote<strong>in</strong> nutrition can be determ<strong>in</strong>ed by measur<strong>in</strong>g the dietary <strong>in</strong>take<br />
<strong>and</strong> output <strong>of</strong> nitrogenous compounds from the body. Although nucleic acids also<br />
conta<strong>in</strong> nitrogen, prote<strong>in</strong> is the major dietary source <strong>of</strong> nitrogen <strong>and</strong> measurement <strong>of</strong><br />
total nitrogen <strong>in</strong>take gives a good estimate <strong>of</strong> prote<strong>in</strong> <strong>in</strong>take (mg N Ч 6.25 = mg prote<strong>in</strong>,<br />
as nitrogen is 16% <strong>of</strong> most prote<strong>in</strong>s). The output <strong>of</strong> nitrogen from the body is ma<strong>in</strong>ly <strong>in</strong><br />
urea <strong>and</strong> smaller quantities <strong>of</strong> other compounds <strong>in</strong> ur<strong>in</strong>e <strong>and</strong> undigested prote<strong>in</strong> <strong>in</strong> feces,<br />
<strong>and</strong> significant amounts may also be lost <strong>in</strong> sweat <strong>and</strong> shed sk<strong>in</strong>.<br />
The difference between <strong>in</strong>take <strong>and</strong> output <strong>of</strong> nitrogenous compounds is known as<br />
nitrogen balance. Three states can be def<strong>in</strong>ed: In a healthy adult, nitrogen balance is <strong>in</strong><br />
equilibrium when <strong>in</strong>take equals output, <strong>and</strong> there is no change <strong>in</strong> the total body content<br />
<strong>of</strong> prote<strong>in</strong>. In a grow<strong>in</strong>g child, a pregnant woman, or <strong>in</strong> recovery from prote<strong>in</strong> loss, the<br />
excretion <strong>of</strong> nitrogenous compounds is less than the dietary <strong>in</strong>take <strong>and</strong> there is net<br />
retention <strong>of</strong> nitrogen <strong>in</strong> the body as prote<strong>in</strong>, ie, positive nitrogen balance. In response<br />
to trauma or <strong>in</strong>fection or if the <strong>in</strong>take <strong>of</strong> prote<strong>in</strong> is <strong>in</strong>adequate to meet requirements<br />
there is net loss <strong>of</strong> prote<strong>in</strong> nitrogen from the body, ie, negative nitrogen balance. The<br />
cont<strong>in</strong>ual catabolism <strong>of</strong> tissue prote<strong>in</strong>s creates the requirement for dietary prote<strong>in</strong> even<br />
<strong>in</strong> an adult who is not grow<strong>in</strong>g, though some <strong>of</strong> the am<strong>in</strong>o acids released can be<br />
reutilized.<br />
Nitrogen balance studies show that the average daily requirement is 0.6 g <strong>of</strong><br />
prote<strong>in</strong> per kilogram <strong>of</strong> body weight (the factor 0.75 should be used to allow for<br />
<strong>in</strong>dividual variation), or approximately 50 g/d. Average <strong>in</strong>takes <strong>of</strong> prote<strong>in</strong> <strong>in</strong> developed<br />
countries are about 80–100 g/d, ie, 14–15% <strong>of</strong> energy <strong>in</strong>take. Because grow<strong>in</strong>g children<br />
are <strong>in</strong>creas<strong>in</strong>g the prote<strong>in</strong> <strong>in</strong> the body, they have a proportionately greater requirement<br />
than adults <strong>and</strong> should be <strong>in</strong> positive nitrogen balance. Even so, the need is relatively
small compared with the requirement for prote<strong>in</strong> turnover. In some countries, prote<strong>in</strong><br />
<strong>in</strong>take may be <strong>in</strong>adequate to meet these requirements, result<strong>in</strong>g <strong>in</strong> stunt<strong>in</strong>g <strong>of</strong> growth.<br />
Residual nitrogen – nonprote<strong>in</strong> nitrogen, that is nitrogen <strong>of</strong> organic <strong>and</strong><br />
<strong>in</strong>organic compounds that rema<strong>in</strong> <strong>in</strong> <strong>blood</strong> after prote<strong>in</strong> sedimentation.<br />
Organic <strong>and</strong> <strong>in</strong>organic compounds <strong>of</strong> residual nitrogen are as follows: urea (50 %<br />
<strong>of</strong> the residual nitrogen), am<strong>in</strong>o acids (25 %), creat<strong>in</strong>e <strong>and</strong> creat<strong>in</strong><strong>in</strong>e (7,5 %), salts <strong>of</strong><br />
ammonia <strong>and</strong> <strong>in</strong>dicane (0,5 %), other compounds (about 13 %).<br />
Urea is formed <strong>in</strong> liver dur<strong>in</strong>g the degradation <strong>of</strong> am<strong>in</strong>o acids, pyrimid<strong>in</strong>e<br />
nucleotides <strong>and</strong> other nitrogen conta<strong>in</strong><strong>in</strong>g compounds. Am<strong>in</strong>o acids are formed as result<br />
<strong>of</strong> prote<strong>in</strong> decomposition or ow<strong>in</strong>g to the conversion <strong>of</strong> fatty acids or carbohydrates to<br />
am<strong>in</strong>o acids. The pool <strong>of</strong> am<strong>in</strong>o acids <strong>in</strong> <strong>blood</strong> is also supported by the process <strong>of</strong> their<br />
absorption <strong>in</strong> <strong>in</strong>test<strong>in</strong>e. Creat<strong>in</strong>e is produced <strong>in</strong> kidneys <strong>and</strong> liver from am<strong>in</strong>o acids<br />
glyc<strong>in</strong>e <strong>and</strong> arg<strong>in</strong><strong>in</strong>e, creat<strong>in</strong><strong>in</strong>e is formed <strong>in</strong> muscles as result <strong>of</strong> creat<strong>in</strong>e phosphate<br />
splitt<strong>in</strong>g. In result <strong>of</strong> ammonia neutralization the ammonia salts can be formed. Indicane<br />
is the product <strong>of</strong> <strong>in</strong>dol neutralization <strong>in</strong> the liver.
Creat<strong>in</strong><strong>in</strong>e Ur<strong>in</strong>e<br />
The content <strong>of</strong> residual nitrogen <strong>in</strong> <strong>blood</strong> is 0,2 – 0,4 g/l.<br />
The pathways <strong>of</strong> convertion <strong>of</strong> am<strong>in</strong>o acid nonnitrogen residues.<br />
The removal <strong>of</strong> the am<strong>in</strong>o group <strong>of</strong> an am<strong>in</strong>o acid by transam<strong>in</strong>ation or<br />
oxidative deam<strong>in</strong>ation produces an α-keto acid that conta<strong>in</strong>s the carbon skeleton<br />
from the am<strong>in</strong>o acid (nonnitrogen residues). These α-keto acids can be used for the<br />
biosynthesis <strong>of</strong> non-essential am<strong>in</strong>o acids or undergoes a different degradation<br />
process. For alan<strong>in</strong>e <strong>and</strong> ser<strong>in</strong>e, the degradation requires a s<strong>in</strong>gle step. For most<br />
carbon arrangements, however, multistep reaction sequences are required. There
are only seven degradation sequences for 20 am<strong>in</strong>o acids. The seven degradation<br />
products are pyruvate, acetyl CoA, acetoacetyl CoA, α-ketoglutarate, succ<strong>in</strong>yl<br />
CoA, fumarate, <strong>and</strong> oxaloacetate. The last four products are <strong>in</strong>termediates <strong>in</strong> the<br />
citric acid cycle. Some am<strong>in</strong>o acids have more than one pathway for degradation.<br />
The major po<strong>in</strong>t <strong>of</strong> entry <strong>in</strong>to the tricarboxylate cycle is via acetyl-CoA; 10 am<strong>in</strong>o<br />
acids enter by this route. Of these, six (alan<strong>in</strong>e, glyc<strong>in</strong>e, ser<strong>in</strong>e, threon<strong>in</strong>e, tryptophan<br />
<strong>and</strong> cyste<strong>in</strong>e) are degraded to acetyl-CoA via pyruvate, five (phenylalan<strong>in</strong>e, tyros<strong>in</strong>e,<br />
leuc<strong>in</strong>e, lys<strong>in</strong>e, <strong>and</strong> tryptophan) are degraded via acetoacetyl-CoA, <strong>and</strong> three<br />
(isoleuc<strong>in</strong>e, leuc<strong>in</strong>e <strong>and</strong> tryptophan) yield acetyl-CoA directly. Leuc<strong>in</strong>e <strong>and</strong> tryptophan<br />
yield both acetoacetyl-CoA <strong>and</strong> acetyl-CoA as end products.<br />
The carbon skeletons <strong>of</strong> five am<strong>in</strong>o acids (arg<strong>in</strong><strong>in</strong>e, histid<strong>in</strong>e, glutamate, glutam<strong>in</strong>e<br />
<strong>and</strong> prol<strong>in</strong>e) enter the tricarboxylic acid cycle via -ketoglutarate.<br />
The carbon skeletons <strong>of</strong> methion<strong>in</strong>e, isoleuc<strong>in</strong>e, <strong>and</strong> val<strong>in</strong>e are ultimately degraded<br />
via propionyl-CoA <strong>and</strong> methyl-malonyl-CoA to succ<strong>in</strong>yl-CoA; these am<strong>in</strong>o acids are<br />
thus glycogenic.<br />
Fumarate is formed <strong>in</strong> catabolism <strong>of</strong> phenylalan<strong>in</strong>e, aspartate <strong>and</strong> tyros<strong>in</strong>e.<br />
Oxaloacetate is formed <strong>in</strong> catabolism <strong>of</strong> aspartate <strong>and</strong> asparag<strong>in</strong>e. Aspartate is<br />
converted to the oxaloacetate by transam<strong>in</strong>ation.<br />
Am<strong>in</strong>o acids that are degraded to citric acid cycle <strong>in</strong>termediates can serve as<br />
glucose precursors <strong>and</strong> are called glucogenic. A glucogenic am<strong>in</strong>o acid is an am<strong>in</strong>o<br />
acid whose carbon-conta<strong>in</strong><strong>in</strong>g degradation product(s) can be used to produce glucose<br />
via gluconeogenesis.<br />
Am<strong>in</strong>o acids that are degraded to acetyl CoA or acetoacetyl CoA can<br />
contribute to the formation <strong>of</strong> fatty acids or ketone bodies <strong>and</strong> are called<br />
ketogenic. A ketogenic am<strong>in</strong>o acid is an am<strong>in</strong>o acid whose carbon-conta<strong>in</strong><strong>in</strong>g<br />
degradation product(s) can be used to produce ketone bodies.<br />
Am<strong>in</strong>o acids that are degraded to pyruvate can be either glucogenic or ketogenic.<br />
Pyruvate can be metabolized to either oxaloacetate (glucogenic) or acetyl CoA<br />
(ketogenic).
Only two am<strong>in</strong>o acids are purely ketogenic: leuc<strong>in</strong>e <strong>and</strong> lys<strong>in</strong>e. N<strong>in</strong>e am<strong>in</strong>o acids<br />
are both glucogenic <strong>and</strong> ketogenic: those degraded to pyruvate (alan<strong>in</strong>e, glyc<strong>in</strong>e,<br />
cyste<strong>in</strong>e, ser<strong>in</strong>e, threon<strong>in</strong>e, tryptophan), as well as tyros<strong>in</strong>e, phenylalan<strong>in</strong>e, <strong>and</strong><br />
isoleuc<strong>in</strong>e (which have two degradation products). The rema<strong>in</strong><strong>in</strong>g n<strong>in</strong>e am<strong>in</strong>o acids are<br />
purely glucogenic (arg<strong>in</strong><strong>in</strong>e, asparag<strong>in</strong>e, aspartate, glutam<strong>in</strong>e, glutamate, val<strong>in</strong>e,<br />
histid<strong>in</strong>e, methion<strong>in</strong>e, prol<strong>in</strong>e)<br />
The regulation <strong>of</strong> prote<strong>in</strong> metabolism. Prote<strong>in</strong> metabolism is regulated by different<br />
hormones. All hormones accord<strong>in</strong>g to their action on prote<strong>in</strong> synthesis or splitt<strong>in</strong>g are<br />
divided on two groups: anabolic <strong>and</strong> catabolic. Anabolic hormones promote to the<br />
prote<strong>in</strong> synthesis. Catabolic hormones enhance the decomposition <strong>of</strong> prote<strong>in</strong>s.<br />
Somatotropic hormone (STH, growth hormone):<br />
- stimulates the pass<strong>in</strong>g <strong>of</strong> am<strong>in</strong>o acids <strong>in</strong>to the cells;<br />
- activates the synthesis <strong>of</strong> prote<strong>in</strong>s, DNA, RNA.<br />
Thyrox<strong>in</strong>e <strong>and</strong> triiodthyron<strong>in</strong>e:<br />
- <strong>in</strong> <strong>normal</strong> concentration stimulate the synthesis <strong>of</strong> prote<strong>in</strong>s <strong>and</strong><br />
nucleic acids;<br />
Insul<strong>in</strong>:<br />
Glucagon:<br />
Ep<strong>in</strong>ephr<strong>in</strong>e:<br />
- <strong>in</strong> excessive concentration activate the catabolic processes.<br />
- <strong>in</strong>creases the permeability <strong>of</strong> cell membranes for am<strong>in</strong>o acids;<br />
- activates synthesis <strong>of</strong> prote<strong>in</strong>s <strong>and</strong> nucleic acids;<br />
- <strong>in</strong>hibits the conversion <strong>of</strong> am<strong>in</strong>o acids <strong>in</strong>to carbohydrates.<br />
- stimulates the conversion <strong>of</strong> am<strong>in</strong>o acids <strong>in</strong>to carbohydrates.<br />
- activates the prote<strong>in</strong> decomposition.<br />
Glucocorticoids:<br />
- stimulate the catabolic processes (prote<strong>in</strong> decomposition) <strong>in</strong><br />
connective, lymphoid <strong>and</strong> muscle tissues <strong>and</strong> activate the processes <strong>of</strong> prote<strong>in</strong><br />
synthesis <strong>in</strong> liver;<br />
- stimulate the activity <strong>of</strong> am<strong>in</strong>otransferases;
azotemia.<br />
Sex hormones:<br />
- activate the synthesis <strong>of</strong> urea.<br />
- stimulate the processes <strong>of</strong> prote<strong>in</strong>, DNA, RNA synthesis;<br />
- cause the positive nitrogenous balance.<br />
The role <strong>of</strong> liver <strong>in</strong> prote<strong>in</strong> metabolism:<br />
– synthesis <strong>of</strong> plasma prote<strong>in</strong>s. Most <strong>of</strong> plasma prote<strong>in</strong>s are<br />
synthesized <strong>in</strong> liver: all album<strong>in</strong>s, 75-90 % <strong>of</strong> α-globul<strong>in</strong>s, 50 % <strong>of</strong> β-<br />
globul<strong>in</strong>s, all prote<strong>in</strong>s <strong>of</strong> <strong>blood</strong> clott<strong>in</strong>g systems (prothromb<strong>in</strong>, fibr<strong>in</strong>ogen,<br />
proconvert<strong>in</strong>, proacceler<strong>in</strong>e). Only γ-globul<strong>in</strong>s are synthesized <strong>in</strong> the cells <strong>of</strong><br />
reticuloendothelial system.<br />
– synthesis <strong>of</strong> urea <strong>and</strong> uric acid;<br />
– synthesis <strong>of</strong> chol<strong>in</strong>e <strong>and</strong> creat<strong>in</strong>e;<br />
– transam<strong>in</strong>ation <strong>and</strong> deam<strong>in</strong>ation <strong>of</strong> am<strong>in</strong>o acids.<br />
Cl<strong>in</strong>ical significance <strong>of</strong> residual nitrogen measurement <strong>in</strong> <strong>blood</strong>. The k<strong>in</strong>ds <strong>of</strong><br />
Azotemia - <strong>in</strong>crease <strong>of</strong> the residual nitrogen content <strong>in</strong> <strong>blood</strong>. There are two k<strong>in</strong>ds<br />
<strong>of</strong> azotemia: absolute <strong>and</strong> relative.<br />
Absolute azotemia – accumulation <strong>of</strong> the <strong>components</strong> <strong>of</strong> residual nitrogen <strong>in</strong><br />
<strong>blood</strong>. Relative azotemia occurs <strong>in</strong> dehydration <strong>of</strong> the organism (diarrhea, vomit<strong>in</strong>g).<br />
Absolute azotemia can be divided on the productive azotemia <strong>and</strong> retention<br />
azotemia. Retention azotemia is caused by the poor excretion <strong>of</strong> the nitrogen conta<strong>in</strong><strong>in</strong>g<br />
compounds via the kidneys; <strong>in</strong> this case the entry <strong>of</strong> nitrogen conta<strong>in</strong><strong>in</strong>g compounds<br />
<strong>in</strong>to the <strong>blood</strong> is <strong>normal</strong>.<br />
Retention azotemia can be divided on the renal <strong>and</strong> extrarenal. Renal retention<br />
azotemia occurs <strong>in</strong> kidney diseases (glomerulonephritis, pyelonephritis, kidney<br />
tuberculosis et c.). Extrarenal retention azotemia is caused by the violations <strong>of</strong> kidney<br />
hemodynamic <strong>and</strong> decrease <strong>of</strong> glomerulus filtration processes (heart failure, local<br />
disorders <strong>of</strong> kidney hemodynamic).<br />
Productive azotemia is conditioned by the enhanced entry <strong>of</strong> nitrogen conta<strong>in</strong><strong>in</strong>g<br />
compounds <strong>in</strong>to the <strong>blood</strong>. The function <strong>of</strong> kidneys <strong>in</strong> this case doesn’t suffer.
Productive azotemia can be observed <strong>in</strong> cachexia, leukoses, malignant tumors,<br />
treatment by glucocorticoids.<br />
Prerenal Azotemia<br />
Alternate Names : Azotemia - Prerenal, Renal Underperfusion, Uremia<br />
Azotemia<br />
From Wikipedia, the free encyclopedia<br />
Jump to: navigation, search<br />
Kidney Anatomy<br />
It has been suggested that this article or section be merged <strong>in</strong>to uremia. (Discuss)<br />
Azotemia is a medical condition characterized by ab<strong>normal</strong> levels <strong>of</strong> urea,<br />
creat<strong>in</strong><strong>in</strong>e, various body waste compounds, <strong>and</strong> other nitrogen-rich compounds <strong>in</strong> the<br />
<strong>blood</strong> as a result <strong>of</strong> <strong>in</strong>sufficient filter<strong>in</strong>g <strong>of</strong> the <strong>blood</strong> by the kidneys.<br />
Uremia can be used as a synonym, or can be used to <strong>in</strong>dicate severe azotemia, <strong>in</strong><br />
which symptoms are produced.
Azotemia can be classified accord<strong>in</strong>g to its cause. In prerenal azotemia the <strong>blood</strong><br />
supply to the kidneys is <strong>in</strong>adequate. In postrenal azotemia the ur<strong>in</strong>ary outflow tract is<br />
obstructed. Other forms <strong>of</strong> azotemia are caused by diseases <strong>of</strong> the kidneys themselves.<br />
Other causes <strong>of</strong> azotemia <strong>in</strong>clude congestive heart failure, shock, severe burns,<br />
prolonged vomit<strong>in</strong>g or diarrhea, some antiviral medications, liver failure, or trauma to<br />
the kidney(s).<br />
[edit] Signs <strong>and</strong> symptoms (prerenal azotemia)<br />
position)<br />
Decreased or absent ur<strong>in</strong>e output<br />
Fatigue<br />
Decreased alertness<br />
Confusion<br />
Pale sk<strong>in</strong> color<br />
Rapid pulse<br />
Dry mouth<br />
Thirst, swell<strong>in</strong>g (edema, anasarca)<br />
Orthostatic <strong>blood</strong> pressure (rises or falls, significantly depend<strong>in</strong>g on<br />
A ur<strong>in</strong>alysis will typically show a decreased ur<strong>in</strong>e sodium level, a high ur<strong>in</strong>e<br />
creat<strong>in</strong><strong>in</strong>e-to- serum creat<strong>in</strong><strong>in</strong>e ratio, a high ur<strong>in</strong>e urea-to-serum urea ratio, <strong>and</strong><br />
concentrated ur<strong>in</strong>e (determ<strong>in</strong>ed by osmolality <strong>and</strong> specific gravity). None <strong>of</strong> these is<br />
particularly useful <strong>in</strong> diagnosis.<br />
Prompt treatment <strong>of</strong> some causes <strong>of</strong> azotemia can result <strong>in</strong> restoration <strong>of</strong> kidney<br />
function; delayed treatment may result <strong>in</strong> permanent loss <strong>of</strong> renal function. Treatment<br />
may <strong>in</strong>clude hemodialysis or peritoneal dialysis, medications to <strong>in</strong>crease cardiac output<br />
<strong>and</strong> <strong>in</strong>crease <strong>blood</strong> pressure, <strong>and</strong> the treatment <strong>of</strong> the condition that caused the azotemia<br />
to beg<strong>in</strong> with. NOTE: Azotemia is not diagnosed with ab<strong>normal</strong>ly high levels <strong>of</strong><br />
Creat<strong>in</strong><strong>in</strong>e. Azotemia simply refers to an elevated level <strong>of</strong> urea <strong>in</strong> the <strong>blood</strong>.
Added Note: Uremia is not azotemia. Azotemia is one <strong>of</strong> many cl<strong>in</strong>ical<br />
characteristics <strong>of</strong> uremia, which is a syndome characteristic <strong>of</strong> renal disease. Uremia<br />
<strong>in</strong>cludes Azotemia, as well as acidosis, hyperkalemia, hypertension, anemia <strong>and</strong><br />
hypocalcemia along with other f<strong>in</strong>d<strong>in</strong>gs.<br />
Retrieved from "http://en.wikipedia.org/wiki/Azotemia"<br />
PATIENT HISTORY:<br />
The patient is a 60 year old male with a previous history <strong>of</strong> thoracic aortic<br />
aneurysm. Currently presents with an abdom<strong>in</strong>al aortic aneurysm <strong>and</strong> liver <strong>and</strong> kidney<br />
masses. Admitted for liver <strong>and</strong> kidney transplant.<br />
The specimen is received unfixed <strong>and</strong> <strong>in</strong> three parts.<br />
Part I:<br />
Part 1 is labeled "liver" <strong>and</strong> consists <strong>of</strong> a 33.6 pound native hepatectomy, 50.0 x<br />
47.0 x 17.0 cm. The capsular surface is tan <strong>and</strong> polycystic with cysts rang<strong>in</strong>g from 0.3<br />
to 11.0 cm. <strong>in</strong> greatest dimension. The capsular surfaces are p<strong>in</strong>k to p<strong>in</strong>k-gray <strong>and</strong><br />
occupy approximately 95% <strong>of</strong> the liver surface. The caudate lobe is 15.0 x 9.0 x 8.0 cm.<br />
<strong>and</strong> is also almost entirely occupied by cysts. There is no adjacent <strong>in</strong>ferior vena cava.<br />
The associated hepatic ve<strong>in</strong>s are p<strong>in</strong>k-gray <strong>and</strong> free <strong>of</strong> obstruction. The attached<br />
gallbladder is 21.0 x 3.0 x 2.5 cm. with a p<strong>in</strong>k- gray, smooth serosal surface. The<br />
gallbladder conta<strong>in</strong>s approximately 20 cc. <strong>of</strong> a viscus green bile. It is 6.0 cm. <strong>in</strong> open
circumference <strong>and</strong> has a wall thickness <strong>of</strong> approximately 0.1 cm. The mucosal surface<br />
is trabecular, tan <strong>and</strong> hemorrhagic. The 1.5 cm. <strong>of</strong> attached cystic duct is free <strong>of</strong><br />
obstruction <strong>and</strong> 2.0 cm. <strong>in</strong> open circumference. On dissection <strong>of</strong> the superficial hilus,<br />
patent portal ve<strong>in</strong>, hepatic arteries <strong>and</strong> hepatic duct are noted. The cut surface consists<br />
<strong>of</strong> approximately 5% tan, congested hepatic parenchyma. The rema<strong>in</strong>der consist<strong>in</strong>g <strong>of</strong><br />
p<strong>in</strong>k- gray to p<strong>in</strong>k-purple, fluid filled cysts, rang<strong>in</strong>g from 0.3 to 11.0 cm. <strong>in</strong> greatest<br />
dimension. Some <strong>of</strong> the larger cysts also conta<strong>in</strong> a gray-green, necrotic material.<br />
Representative tissue is frozen <strong>in</strong> bulk, submitted for outside research purposes <strong>and</strong><br />
stocked <strong>in</strong> formal<strong>in</strong>. Gross photographs are taken <strong>and</strong> sections submitted.<br />
Part II:<br />
Part 2 is labeled "left kidney" <strong>and</strong> consists <strong>of</strong> a 1,430 gram native nephrectomy,<br />
24.0 x 11.0 x 9.0 cm. with attached fat <strong>and</strong> Gerota's fascia. The cortical surface is<br />
almost entirely obliterated by p<strong>in</strong>k-gray <strong>and</strong> hemorrhagic cysts, rang<strong>in</strong>g from 0.1 to 5.0<br />
cm. <strong>in</strong> greatest dimension. The kidney has been previously sagittally sectioned,<br />
expos<strong>in</strong>g a cut surface consist<strong>in</strong>g <strong>of</strong> approximately less than 5% <strong>of</strong> identifiable renal<br />
cortex at the anterior <strong>and</strong> posterior aspects. The rema<strong>in</strong>der <strong>of</strong> the kidney consists <strong>of</strong><br />
fluid filled cysts rang<strong>in</strong>g from 0.1 to 5.0 cm. <strong>in</strong> greatest dimension <strong>and</strong> p<strong>in</strong>k-white,<br />
grossly unremarkable renal pelvis. Some necrotic, gray-green material is conta<strong>in</strong>ed<br />
with<strong>in</strong> the larger cyst. The hilar structures consists <strong>of</strong> patent artery ve<strong>in</strong> <strong>and</strong> ureter. The<br />
ureter, 8.0 cm. <strong>in</strong> length, has an irregular p<strong>in</strong>k-gray mucosal surface with some areas<br />
resembl<strong>in</strong>g diverticula. Representative tissue is frozen <strong>in</strong> bulk, submitted <strong>in</strong><br />
Karnovsky's fixative for possible electron microscopic evaluation <strong>and</strong> stocked <strong>in</strong><br />
formal<strong>in</strong>. Gross photos are taken <strong>and</strong> sections submitted.
Part III:<br />
Part 3 is labeled "spleen" <strong>and</strong> consists <strong>of</strong> a 130 gram, purple- gray spleen, 11.0 x<br />
7.0 x 4.0 cm. Torn cauterized capsule is evident at the <strong>in</strong>ferior aspect. A 5.0 x 4.0 x 3.0<br />
cm. bulge is situated at the convexity. On cross section, a 3.0 x 2.0 x 2.0 cm. fluid<br />
filled, multilocular cyst is surrounded by red-brown, splenic parenchyma <strong>and</strong> patent<br />
malpighian corpuscles. Two separate cysts, 0.3 to 0.6 cm. are adjacent to the ma<strong>in</strong> cyst.<br />
No other lesions are noted. The hilus consists <strong>of</strong> patent vasculature with surround<strong>in</strong>g<br />
yellow <strong>and</strong> hemorrhagic fat. Gross photos are taken <strong>and</strong> representative sections <strong>of</strong><br />
spleen with previously mentioned cysts are submitted <strong>in</strong> cassettes 3A through 3D.<br />
MICROSCOPIC DESCRIPTION<br />
MICROSCOPIC DESCRIPTION:<br />
Factor VIII<br />
Sections from the liver show extensive cyst formation affect<strong>in</strong>g more than 90% <strong>of</strong><br />
the liver parenchyma. The limited amount <strong>of</strong> liver tissue which rema<strong>in</strong>s shows a variety<br />
<strong>of</strong> changes vary<strong>in</strong>g from atrophy to hemorrhage. Similar epithelium l<strong>in</strong>ed cysts are seen<br />
<strong>in</strong> the kidney. Some cysts have ruptured <strong>and</strong> lead to hemorrhagic necrosis, calcification<br />
<strong>and</strong> fibrosis. Focal cholesterol clefts <strong>and</strong> foreign body giant cells are seen. The spleen<br />
shows an exp<strong>and</strong>ed red pulp <strong>and</strong> multiple cystic spaces l<strong>in</strong>ed by flattened cells.
FINAL DIAGNOSIS<br />
Lipoprote<strong>in</strong>s <strong>and</strong> Apoprote<strong>in</strong>s<br />
http://www.youtube.com/watch?v=97uiV4RiSAY<br />
Lipids are a group <strong>of</strong> fatty substances that <strong>in</strong>cludes triglycerides (fat), phospholipids <strong>and</strong><br />
sterols (e.g. cholesterol). They constitute an important source <strong>of</strong> energy, serve as precursors for<br />
a number <strong>of</strong> essential compounds, <strong>and</strong> are key <strong>components</strong> <strong>of</strong> cells <strong>and</strong> tissues. Cholesterol, for<br />
example, is an <strong>in</strong>dispensable constituent <strong>of</strong> cellular membranes (1), as well as the precursor for<br />
both steroid hormones <strong>and</strong> bile acids. On average, the body utilizes approximately 1000<br />
milligrams <strong>of</strong> cholesterol per day, 30% <strong>of</strong> which comes directly from foods <strong>of</strong> animal orig<strong>in</strong>,<br />
<strong>and</strong> the rest is synthesized <strong>in</strong> the liver. Due to the <strong>in</strong>solubility <strong>of</strong> cholesterol <strong>and</strong> other fatty<br />
compounds <strong>in</strong> the <strong>blood</strong>, their redistribution <strong>in</strong> the body requires specialized carriers capable <strong>of</strong><br />
solubilz<strong>in</strong>g, ferry<strong>in</strong>g, <strong>and</strong> unload<strong>in</strong>g them at specific target sites. Miscarriage <strong>of</strong> lipids while <strong>in</strong><br />
circulation may lead to atherosclerosis; a cl<strong>in</strong>ical condition marked by fatty deposits <strong>in</strong> the <strong>in</strong>ner<br />
walls <strong>of</strong> arteries, <strong>and</strong> the lead<strong>in</strong>g cause <strong>of</strong> death <strong>and</strong> disability <strong>in</strong> Western countries.<br />
Most lipids are transported <strong>in</strong> the <strong>blood</strong> as part <strong>of</strong> soluble complexes called lipoprote<strong>in</strong>s<br />
(LPs). Plasma LPs are spherical particles composed <strong>of</strong> a hydrophobic lipid core surrounded by a<br />
hydrophilic layer, which renders the particles soluble. The lipid core conta<strong>in</strong>s primarily<br />
triglycerides (TG) <strong>and</strong> cholesteryl esters (CE), as well as small amounts <strong>of</strong> other fatty<br />
compounds, such as sph<strong>in</strong>golipids <strong>and</strong> fat-soluble vitam<strong>in</strong>s (e.g. vitam<strong>in</strong>s A, D, E, <strong>and</strong> K). The<br />
external layer is made <strong>of</strong> phospholipids, unesterified cholesterol, <strong>and</strong> specialized prote<strong>in</strong>s, called<br />
apolipoprote<strong>in</strong>s or apoprote<strong>in</strong>s. These prote<strong>in</strong>s facilitate lipid solubilization <strong>and</strong> help to ma<strong>in</strong>ta<strong>in</strong><br />
the structural <strong>in</strong>tegrity <strong>of</strong> LPs. They also serve as lig<strong>and</strong>s for LP receptors <strong>and</strong> regulate the<br />
activity <strong>of</strong> LP metabolic enzymes. As depicted <strong>in</strong> (Figure 1), the amphipathic molecules that<br />
compose the outer layer <strong>of</strong> LPs are arranged so that their hydrophobic parts face the central core,<br />
<strong>and</strong> their hydrophilic regions face the surround<strong>in</strong>g aqueous environment.
Figure 1: Schematic Illustration <strong>of</strong> a Lipoprote<strong>in</strong> Particle<br />
Cholesteryl esters, which do not conta<strong>in</strong> a free hydroxyl group (-OH) are more<br />
hydrophobic than cholesterol, <strong>and</strong> better accommodated <strong>in</strong> the core <strong>of</strong> LPs. The conversion <strong>of</strong><br />
cholesterol to CE is catalyzed by a LP-associated enzyme called lecith<strong>in</strong>-cholesterol<br />
acyltransferase (LCAT). This enzyme, which promotes packag<strong>in</strong>g <strong>of</strong> cholesteryl molecules <strong>in</strong><br />
LPs, is critical for <strong>normal</strong> cholesterol metabolism. Deficiency <strong>of</strong> LCAT activity leads to<br />
accumulation <strong>of</strong> unesterified cholesterol <strong>in</strong> tissues, <strong>and</strong> is associated with a number <strong>of</strong> cl<strong>in</strong>ical<br />
conditions <strong>in</strong>clud<strong>in</strong>g corneal opacity, hemolytic anemia, <strong>and</strong> premature atherosclerosis.<br />
Dur<strong>in</strong>g ord<strong>in</strong>ary metabolism, plasma LPs lose, acquire, <strong>and</strong> exchange their lipid <strong>and</strong><br />
prote<strong>in</strong> constituents. Normally, fat-rich LPs lose most <strong>of</strong> their fat with<strong>in</strong> a few hours <strong>of</strong> food<br />
<strong>in</strong>gestion, <strong>and</strong> become smaller <strong>and</strong> denser particles with higher relative cholesterol content. The<br />
depletion <strong>of</strong> fat from LPs is catalyzed by lipoprote<strong>in</strong> lipase (LPL). This lipolytic enzyme is<br />
located on the surface <strong>of</strong> endothelial capillaries, <strong>and</strong> degrades triglycerides to free fatty acids<br />
(FFAs) <strong>and</strong> glycerol. The released FFAs may stay <strong>in</strong> circulation bound to album<strong>in</strong>, or be taken-<br />
up by muscle <strong>and</strong> fat cells for usage <strong>and</strong> storage, respectively.<br />
Lipids <strong>of</strong> dietary orig<strong>in</strong> are processed by <strong>in</strong>test<strong>in</strong>al epithelial cells, <strong>and</strong> then secreted <strong>in</strong>to<br />
the <strong>blood</strong>stream as part <strong>of</strong> large, fat-rich LPs called chylomicrons (chylo = milky, micron=<br />
<strong>in</strong>dicates particle size). En route to the liver, chylomicrons (CM) pass through endothelial<br />
capillaries, lose some fat, <strong>and</strong> their remnants are taken-up by liver cells. In the liver, the lipids<br />
obta<strong>in</strong>ed from CM remnants are re-processed <strong>and</strong> then secreted back <strong>in</strong>to the <strong>blood</strong>stream as
part <strong>of</strong> very low-density LPs (VLDL). Depletion <strong>of</strong> fat from VLDL transforms the particle <strong>in</strong>to<br />
an <strong>in</strong>termediate density lipoprote<strong>in</strong> (IDL), which upon further degradation <strong>of</strong> its fat is converted<br />
<strong>in</strong>to a relatively stable particle, called low density lipoprote<strong>in</strong> (LDL). Because <strong>of</strong> its high<br />
cholesterol content, LDL is also called LDL-cholesterol. Of the total <strong>blood</strong> cholesterol, 60-75%<br />
is found <strong>in</strong> LDL <strong>and</strong> the rest primarily <strong>in</strong> high-density lipoprote<strong>in</strong> (HDL) particles. The ma<strong>in</strong><br />
characteristics <strong>of</strong> plasma LPs <strong>and</strong> their associated apoprote<strong>in</strong>s are summarized <strong>in</strong> (Tables I <strong>and</strong><br />
II), respectively.<br />
All peripheral cells express the LDL-receptor (LDLR), <strong>and</strong> recycle it to the cell surface<br />
upon need for cholesterol. Cholesterol is delivered to these cells through b<strong>in</strong>d<strong>in</strong>g <strong>of</strong> LDL to<br />
LDLR, which triggers endocytosis (<strong>in</strong>ternalization) <strong>of</strong> both species. When the need for<br />
cholesterol is satisfied, the recycl<strong>in</strong>g <strong>of</strong> LDLR is discont<strong>in</strong>ued. Normally, an LDL particle stays<br />
<strong>in</strong> circulation for no more than a few days before be<strong>in</strong>g consumed by a cholesterol need<strong>in</strong>g cell.<br />
However, under conditions <strong>of</strong> susta<strong>in</strong>ed cholesterol excess, the particle stays <strong>in</strong> circulation for<br />
longer periods <strong>of</strong> time, <strong>and</strong> becomes more vulnerable to undesired modifications (e.g.<br />
oxidation). As high levels <strong>of</strong> oxidized LDL are commonly found <strong>in</strong> atherosclerotic plaques, they<br />
are thought to be the major <strong>in</strong>ducer <strong>of</strong> atherosclerotic lesions. Hence, LDL became known as
ad cholesterol. However, today we know that not all LDL particles are bad, <strong>and</strong> that some LDL<br />
particles, especially very large ones (with diameter >21.3nm), may even provide protection<br />
aga<strong>in</strong>st atherosclerosis (2). LDL <strong>and</strong> HDL particle sizes are largely determ<strong>in</strong>ed by a LP-<br />
associated prote<strong>in</strong>, called CETP (cholesteryl ester transfer prote<strong>in</strong>). This prote<strong>in</strong> enhances<br />
exchange <strong>of</strong> non-polar lipids, primarily CE <strong>and</strong> TG, <strong>and</strong> facilitates tight packag<strong>in</strong>g <strong>of</strong> CE with<strong>in</strong><br />
the core <strong>of</strong> the particles. The end result <strong>of</strong> prolonged <strong>and</strong>/or efficient CETP action is smaller<br />
LDL <strong>and</strong> HDL particles. [The LP-anchored CETP can be envisioned as hav<strong>in</strong>g a h<strong>and</strong> that<br />
rotates between the <strong>in</strong>terior <strong>and</strong> exterior <strong>of</strong> the particle <strong>and</strong> capable <strong>of</strong> hold<strong>in</strong>g only one lipid<br />
molecule at a time. Grasp<strong>in</strong>g <strong>of</strong> one molecule releases another <strong>and</strong> vise versa.]<br />
Genetic variation at the human CETP gene generates prote<strong>in</strong>s with vary<strong>in</strong>g degrees <strong>of</strong><br />
activity. For example, a s<strong>in</strong>gle codon variation, from isoleuc<strong>in</strong>e to val<strong>in</strong>e at position 405,<br />
generates a mutant prote<strong>in</strong>, designated I405V, which manifests significantly reduced CETP<br />
activity (3, 4). In a new observational study, Barzilai, N. et al. (2) found that people with<br />
homozygosity for the I405V allele have larger HDL <strong>and</strong> LDL particles, <strong>and</strong> that this genotype is<br />
associated with exceptional longevity <strong>and</strong> a markedly reduced risk <strong>of</strong> coronary artery disease<br />
(CAD). Of the 213 centenarians enrolled <strong>in</strong> the study, 80% had a high proportion <strong>of</strong> large LDL<br />
particles, compared to just 8% <strong>of</strong> the subjects <strong>in</strong> the control group (256 people <strong>in</strong> their 60’s <strong>and</strong><br />
70’s) (2). Interest<strong>in</strong>gly, HDL <strong>and</strong> LDL particle sizes are significantly larger <strong>in</strong> women than <strong>in</strong><br />
men, which may account, at least <strong>in</strong> part, for the longer life expectancies <strong>of</strong> women.<br />
Unlike LDL, HDL is not recognized by LDLR, <strong>and</strong> cannot deliver cholesterol to tissue<br />
cells. Instead, it has the ability to remove excess peripheral cholesterol <strong>and</strong> return it to the liver<br />
for recycl<strong>in</strong>g <strong>and</strong> excretion. This process, called reverse cholesterol transport, is thought to<br />
protect aga<strong>in</strong>st atherosclerosis. Observational studies over the last 2 decades have consistently<br />
shown strong correlation between elevated HDL levels <strong>and</strong> low <strong>in</strong>cidents <strong>of</strong> coronary heart<br />
disease (CHD). Hence HDL has been dubbed ―good‖ cholesterol.<br />
HDL is synthesized <strong>in</strong> the liver <strong>and</strong> <strong>in</strong>test<strong>in</strong>e as a nascent, discoid-shaped particle that<br />
conta<strong>in</strong>s predom<strong>in</strong>antly apoA-I, <strong>and</strong> some phospholipids. Upon maturation, HDL assumes a<br />
spherical shape, <strong>and</strong> the composition <strong>of</strong> its core lipids becomes very similar to that <strong>of</strong> LDL.<br />
However, the relative higher prote<strong>in</strong> content <strong>in</strong> HDL renders the particle denser <strong>and</strong> more<br />
resistant to undesired modifications. Unlike the case <strong>of</strong> LDL, the clearance <strong>of</strong> HDL from<br />
circulation is not negatively affected by excess cholesterol, which may be another reason why
HDL, despite be<strong>in</strong>g much smaller particle than LDL (10nm versus 20nm), is not found <strong>in</strong><br />
atherosclerotic plaques. It’s worth not<strong>in</strong>g, that the potential <strong>of</strong> LPs to become harmful is also<br />
<strong>in</strong>fluenced by the character <strong>of</strong> their lipid constituents. For example, vitam<strong>in</strong> E <strong>and</strong> lipids<br />
conta<strong>in</strong><strong>in</strong>g omega-3 fatty acid moieties appear to protect the particles from harmful oxidation<br />
<strong>and</strong> from gett<strong>in</strong>g stuck on the walls <strong>of</strong> <strong>blood</strong> vessels.<br />
The functional difference between LDL <strong>and</strong> HDL results primarily from the different<br />
character <strong>of</strong> their major apoprote<strong>in</strong>s, apoB-100 <strong>and</strong> apoA-I, respectively. ApoB-100, which is<br />
found <strong>in</strong> VLDL, IDL, <strong>and</strong> LDL, but not <strong>in</strong> HDL, serves as a lig<strong>and</strong> for LDLR, <strong>and</strong> provides<br />
LDL with the means to deliver cholesterol to tissue cells. On the other h<strong>and</strong>, apoA-I, which is<br />
found exclusively <strong>in</strong> HDL, has a unique ability to capture <strong>and</strong> solubilze free cholesterol. This<br />
apoA-I ability enables HDL to act as a cholesterol scavenger.<br />
A mutant apoA-I prote<strong>in</strong>, called apoA-I Milano (apoA-Im), has been identified <strong>in</strong> a group<br />
<strong>of</strong> people that live <strong>in</strong> a small village <strong>in</strong> northern Italy (5). Carriers <strong>of</strong> this prote<strong>in</strong>, all<br />
heterozygous for the mutation, had very low levels <strong>of</strong> HDL (7-14 mg/dl) but showed no cl<strong>in</strong>ical<br />
signs <strong>of</strong> atherosclerosis (5-7). HDL particles <strong>in</strong> these subjects were markedly larger than control<br />
(12nm versus 9.4nm), which may account for their immunity aga<strong>in</strong>st premature atherosclerosis.<br />
ApoA-Im differs from natural apoA-I by hav<strong>in</strong>g a cyste<strong>in</strong>e residue at position 173 <strong>in</strong>stead <strong>of</strong><br />
arg<strong>in</strong><strong>in</strong>e. This cyste<strong>in</strong>e residue forms disulfide bridges with other apoA-I molecules or with<br />
apoA-II (6, 7), which apparently lead to larger HDL particles. It also renders apoA-I more<br />
susceptible to catabolism (8), account<strong>in</strong>g for the low HDL levels <strong>in</strong> apoA-Im carriers.<br />
The therapeutic potential <strong>of</strong> apoA-I has been recently assessed <strong>in</strong> patients with acute<br />
coronary syndromes (9). Of the 47 patients that participated <strong>in</strong> a r<strong>and</strong>omized controlled trial, 36<br />
received 5 weekly <strong>in</strong>fusions <strong>of</strong> recomb<strong>in</strong>ant apoA-Im/phospholipid complexes, <strong>and</strong> 11 received<br />
only sal<strong>in</strong>e <strong>in</strong>fusions. The results showed significant regression <strong>in</strong> coronary atherosclerotic<br />
volume <strong>in</strong> the apoA-Im treated group, <strong>and</strong> virtually no change <strong>in</strong> the control group (9). These<br />
results, if reproduced <strong>in</strong> larger cl<strong>in</strong>ical trials, may constitute a revolutionary breakthrough <strong>in</strong> the<br />
non-<strong>in</strong>vasive treatment <strong>of</strong> cardiovascular disease. They should also encourage further<br />
exploration <strong>in</strong>to the therapeutic usefulness <strong>of</strong> apoA-Im <strong>and</strong> <strong>normal</strong> apoA-I <strong>in</strong> manag<strong>in</strong>g<br />
atherosclerotic vascular diseases.<br />
What are lipoprote<strong>in</strong>s?<br />
e LDL (low density lipoprote<strong>in</strong>s) <strong>and</strong> HDL (high density lipoprote<strong>in</strong>s).
Lipid Levels<br />
The lilipoprote<strong>in</strong>s - Any <strong>of</strong> the series <strong>of</strong> soluble lipid-prote<strong>in</strong> complexes which are<br />
transported <strong>in</strong> the <strong>blood</strong>; each aggregate particle consists <strong>of</strong> a spherical hydrophobic<br />
core conta<strong>in</strong><strong>in</strong>g triglycerides <strong>and</strong> cholesterol esters surrounded by an amphipathic<br />
monolayer <strong>of</strong> phopholipids, cholesterol <strong>and</strong> apolipoprote<strong>in</strong>s; classes <strong>of</strong> lipoprote<strong>in</strong>s<br />
<strong>in</strong>clude chylomicrons, very low-density lipoprote<strong>in</strong>s (VLDL), <strong>in</strong>termediate-density<br />
lipoprote<strong>in</strong>s (IDL), low-density lipoprote<strong>in</strong>s (LDL), <strong>and</strong> high-density lipoprote<strong>in</strong>s<br />
(HDL).<br />
chylomicrons - The class <strong>of</strong> largest diameter soluble lipid-prote<strong>in</strong> complexes<br />
which the lowest <strong>in</strong> density (mass to volume ratio); their composition is ~2%<br />
apolipoprote<strong>in</strong>s, ~5% cholesterol, <strong>and</strong> ~93% triglycerides <strong>and</strong> phospholipids; their<br />
<strong>normal</strong> role is to be synthesized by the <strong>in</strong>test<strong>in</strong>al mucosal cells to transport dietary<br />
(exogenous) triglycerides <strong>and</strong> other lipids from the <strong>in</strong>test<strong>in</strong>es via the lacteals <strong>and</strong><br />
lymphatic system to the systemic circulation to the adipose tissue <strong>and</strong> liver for storage<br />
<strong>and</strong> use; they are only present <strong>in</strong> the <strong>blood</strong> <strong>in</strong> significant quantities after the digestion <strong>of</strong><br />
a meal.<br />
low-density lipoprote<strong>in</strong>s (LDL) - The class <strong>of</strong> large diameter soluble lipid-prote<strong>in</strong><br />
complexes which the fourth lowest <strong>in</strong> density (mass to volume ratio); their composition<br />
is ~25% apolipoprote<strong>in</strong>s, ~45% cholesterol, <strong>and</strong> ~30% triglycerides <strong>and</strong> phospholipids;
their <strong>normal</strong> role is to transport cholesterol <strong>and</strong> other lipids from the liver <strong>and</strong> <strong>in</strong>test<strong>in</strong>es<br />
to the tissues for use; elevated levels <strong>of</strong> LDL are associated with <strong>in</strong>creased risk <strong>of</strong><br />
cardiovascular disease. nickname - bad cholesterol<br />
high-density lipoprote<strong>in</strong>s (HDL) - The class <strong>of</strong> small diameter soluble lipid-<br />
prote<strong>in</strong> complexes which the highest <strong>in</strong> density (mass to volume ratio); their<br />
composition is ~45% apolipoprote<strong>in</strong>s, ~25% cholesterol, <strong>and</strong> ~30% triglycerides <strong>and</strong><br />
phospholipids; their <strong>normal</strong> role is to transport cholesterol <strong>and</strong> other lipids from the<br />
tissues to the liver for disposal; elevated levels <strong>of</strong> HDL are associated with decreased<br />
risk <strong>of</strong> cardiovascular disease.<br />
very low-density lipoprote<strong>in</strong>s (VLDL) - The class <strong>of</strong> very large diameter soluble<br />
lipid-prote<strong>in</strong> complexes which the second lowest <strong>in</strong> density (mass to volume ratio);<br />
their composition is ~10% apolipoprote<strong>in</strong>s, ~40% cholesterol, <strong>and</strong> ~50% triglycerides<br />
<strong>and</strong> phospholipids; their <strong>normal</strong> role is to transport triglycerides <strong>and</strong> other lipids from<br />
the liver <strong>and</strong> <strong>in</strong>test<strong>in</strong>es to the tissues for use; elevated levels <strong>of</strong> VLDL are associated<br />
with some <strong>in</strong>creased risk <strong>of</strong> cardiovascular disease.<br />
formation <strong>of</strong> lipoprote<strong>in</strong>s
http://www.youtube.com/watch?v=97uiV4RiSAY<br />
What is Cholesterol?<br />
Cholesterol is a waxy fat found <strong>in</strong> the body <strong>and</strong>, despite what you may have been<br />
told, is a necessary nutrient for the body. Cholesterol is used <strong>in</strong> the formation <strong>of</strong> cell<br />
membranes <strong>and</strong> plays an important role <strong>in</strong> hormone, bile <strong>and</strong> vitam<strong>in</strong> D production.<br />
Cholesterol comes from two sources: the foods that we eat, such as meat, dairy products<br />
<strong>and</strong> eggs, <strong>and</strong> our own liver, which produces about eighty percent <strong>of</strong> all the cholesterol<br />
<strong>in</strong> the body. That means that only about twenty percent <strong>of</strong> our total cholesterol is<br />
obta<strong>in</strong>ed from food. S<strong>in</strong>ce cholesterol is not water-soluble, the liver packages the<br />
cholesterol <strong>in</strong>to t<strong>in</strong>y spheres called lipoprote<strong>in</strong>s so that the cholesterol can be
transported through the <strong>blood</strong>. The lipoprote<strong>in</strong>s can be divided <strong>in</strong>to two different<br />
categories: low density <strong>and</strong> high density lipoprote<strong>in</strong>s.<br />
http://www.youtube.com/watch?v=-WhADd1GKtA&feature=relmfu<br />
Low density lipoprote<strong>in</strong> (LDL): LDL, <strong>of</strong>ten dubbed the "bad" cholesterol, carries<br />
most <strong>of</strong> the cholesterol <strong>in</strong> the <strong>blood</strong> <strong>and</strong> seems to play a role <strong>in</strong> the deposition <strong>of</strong> fat <strong>in</strong><br />
arteries. These deposits result <strong>in</strong> blockages called plaque. In addition to narrow<strong>in</strong>g the<br />
arteries <strong>and</strong> <strong>in</strong>creas<strong>in</strong>g <strong>blood</strong> pressure, plaque contributes to the harden<strong>in</strong>g <strong>of</strong> artery<br />
walls, a condition known as atherosclerosis.<br />
High density lipoprote<strong>in</strong> (HDL): HDL is known as the "good" cholesterol. HDL<br />
carries cholesterol from the <strong>blood</strong> back to the liver for elim<strong>in</strong>ation. It is also responsible<br />
for remov<strong>in</strong>g the plaque buildup along the artery walls. Elevated levels <strong>of</strong> HDL are very<br />
desirable because it helps to clear blockages <strong>in</strong> the arteries, reduces LDL <strong>and</strong> decreases<br />
<strong>blood</strong> pressure.<br />
What are Triglycerides?<br />
Triglycerides are lipids <strong>normal</strong>ly found <strong>in</strong> <strong>in</strong>creased levels <strong>in</strong> the <strong>blood</strong> follow<strong>in</strong>g<br />
the digestion <strong>of</strong> fats <strong>in</strong> the <strong>in</strong>test<strong>in</strong>e. Consumed calories that are not immediately used<br />
are stored <strong>in</strong> fat cells <strong>in</strong> the form <strong>of</strong> triglycerides <strong>and</strong> are later released from fatty tissues<br />
when the body needs energy between meals. The major transporter <strong>of</strong> triglycerides is a<br />
forerunner <strong>of</strong> LDL, a simpler molecule known as VLDL (very low density lipoprote<strong>in</strong>).<br />
As the VLDL loses triglycerides, the VLDL particle is converted <strong>in</strong>to <strong>in</strong>termediate <strong>and</strong><br />
then low density lipoprote<strong>in</strong>. Over time, elevated triglyceride levels may result <strong>in</strong>
pancreatitis—a condition that can cause malabsorption <strong>of</strong> nutrients <strong>and</strong> lead to diabetes.<br />
As pancreatitis progresses, damage can spread to other organs, <strong>in</strong>clud<strong>in</strong>g the heart,<br />
lungs <strong>and</strong> kidneys. High triglyceride levels also promote the deposition <strong>of</strong> cholesterol <strong>in</strong><br />
the arteries <strong>and</strong> are associated with known risk factors for heart disease. The exact role<br />
that triglycerides play as an <strong>in</strong>dependent risk factor is not yet clear because people with<br />
high LDL <strong>and</strong> low HDL levels also have high triglyceride levels.<br />
Although These Researchers Beg to Differ…<br />
One study by Koren-Morag, Graff <strong>and</strong> Goldbourt, published <strong>in</strong> the American<br />
Heart Association journal Circulation, found that <strong>in</strong>dividuals with elevated triglyceride<br />
levels have a nearly thirty percent <strong>in</strong>creased probability <strong>of</strong> suffer<strong>in</strong>g a stroke, even after<br />
tak<strong>in</strong>g <strong>in</strong>to account other risk factors such as cholesterol levels. One <strong>of</strong> the most<br />
important aspects <strong>of</strong> the study is that it clarifies the <strong>in</strong>dependent l<strong>in</strong>k <strong>of</strong> triglyceride<br />
levels to stroke, mean<strong>in</strong>g that a causal relationship is likely.<br />
What is Plaque?<br />
Excess LDL cholesterol cl<strong>in</strong>gs to arterial walls as it is transported through the<br />
system. Macrophages eat the LDL <strong>and</strong> become "foam cells." The cells eventually<br />
rupture <strong>and</strong> beg<strong>in</strong> to form a lipid layer called plaque. Connective fibers form <strong>in</strong> <strong>and</strong><br />
around the fatty layer, caus<strong>in</strong>g it to harden. Over time, the fibrous layer thickens,<br />
narrow<strong>in</strong>g the arterial pathway. When calcium deposits form a crust, the plaque<br />
becomes brittle <strong>and</strong> is more likely to rupture.
The Problem With Plaque<br />
High <strong>blood</strong> cholesterol levels <strong>in</strong>crease the likelihood that the fat will be deposited<br />
as plaque on the <strong>in</strong>ner surface <strong>of</strong> arterial walls. As these deposits <strong>in</strong>crease, the channel<br />
<strong>of</strong> the artery narrows, contribut<strong>in</strong>g to an <strong>in</strong>crease <strong>in</strong> <strong>blood</strong> pressure. To compensate, the<br />
heart must work harder to pump the same volume <strong>of</strong> <strong>blood</strong> through the narrower<br />
arteries. When the coronary arteries themselves are affected by plaque, the harder<br />
work<strong>in</strong>g heart receives less oxygen, thus <strong>in</strong>creas<strong>in</strong>g the risk <strong>of</strong> heart attack. Plaque also<br />
contributes to harden<strong>in</strong>g <strong>of</strong> the arteries, or atherosclerosis. This loss <strong>of</strong> flexibility <strong>in</strong><br />
arterial walls elevates <strong>blood</strong> pressure, putt<strong>in</strong>g the heart at additional risk. When the<br />
plaque deposits become unstable, they burst, releas<strong>in</strong>g their cholesterol <strong>in</strong>to the<br />
<strong>blood</strong>stream all at once. This can trigger clott<strong>in</strong>g <strong>in</strong> small coronary arteries. When the<br />
artery is completely obstructed, <strong>blood</strong> flow stops <strong>and</strong> a heart attack occurs.<br />
http://www.youtube.com/watch?v=XLLBlBiboJI&feature=related<br />
http://www.youtube.com/watch?v=-WhADd1GKtA&feature=relmfu<br />
What is a Lipoprote<strong>in</strong>?<br />
Lipids, such as triacylglycerols <strong>and</strong> cholesterol esters, are virtually <strong>in</strong>soluble <strong>in</strong><br />
aqueous solution. Therefore, lipids must be transported by the circulation <strong>in</strong><br />
COMPLEX WITH water-soluble PROTEINS.<br />
This complex LIPOPROTEIN is a globular micelle-like particle that consists <strong>of</strong> a<br />
nonpolar core <strong>of</strong> triacylglycerols <strong>and</strong> cholesterol esters surrounded by an amphiphilic<br />
coat<strong>in</strong>g <strong>of</strong> prote<strong>in</strong>, phospholipid, <strong>and</strong> cholesterol.<br />
Here is a diagram <strong>of</strong> Low-Density Lipoprote<strong>in</strong> (LDL) which is approximately<br />
25nm <strong>in</strong> diameter:<br />
http://www.youtube.com/watch?v=x-4ZQaiZry8
motion:<br />
ristic<br />
(g/cm)<br />
You need "Quick Time" Player <strong>and</strong> Plug-In to view this LDL particle <strong>in</strong><br />
video<br />
http://www.youtube.com/watch?v=97uiV4RiSAY<br />
Characteristics <strong>of</strong> Lipoprote<strong>in</strong>s <strong>in</strong> Human Plasma<br />
Characte<br />
Density<br />
Particle<br />
Diameter (nm)<br />
Particle<br />
Mass (kD)<br />
Chylomicrons VLDL IDL<br />
~0.95 ~1.006<br />
75-1200 30-80<br />
400,000<br />
80,000<br />
10,000-<br />
%Prote<strong>in</strong> a 1.5-2.5 5-10<br />
1.00<br />
6-1.019<br />
35<br />
25-<br />
500<br />
0-10,000<br />
20<br />
15-<br />
DL<br />
.019<br />
-<br />
1.06<br />
3<br />
8-25<br />
300<br />
0-25<br />
L<br />
1<br />
1<br />
2<br />
2<br />
HDL<br />
1.063-1.210<br />
5-12<br />
175-360<br />
40-55<br />
%Phosph 7-9 15-20 22 1 20-35
olipids a 5-20<br />
%Free<br />
Cholesterol a<br />
%Triacyl<br />
glycerols b<br />
%Cholest<br />
eryl Esters b<br />
Major<br />
Apolipoprote<strong>in</strong><br />
s<br />
1-3 5-10 8<br />
84-89 50-65 22<br />
3-5 10-15 30<br />
AI,AII,B48,CI<br />
,CII,CIII,E<br />
a Surface Components<br />
b Core Lipids<br />
VLDLy Found <strong>in</strong> Egg Yolk<br />
B100,CI,<br />
CII,CIII,E<br />
B10<br />
0,CIII,E<br />
-10<br />
-10<br />
5-40<br />
100<br />
7<br />
7<br />
3<br />
B<br />
3-4<br />
3-5<br />
12<br />
AI,AII,CI,CI<br />
I,CIII,D,E<br />
"VLDLy" was co<strong>in</strong>ed to signify specific lipoprote<strong>in</strong>s that selectively deposit<br />
triacylglycerol to yolk follicles.<br />
The average size <strong>of</strong> a VLDLy particle is 30nm, whereas a generic VLDL particle<br />
is approximately 70nm.
VLDLy Metabolism<br />
Theoretically, a 17g egg yolk that conta<strong>in</strong>s 2.8g <strong>of</strong> prote<strong>in</strong> would conta<strong>in</strong> 1.4g <strong>of</strong><br />
apoB (49% total yolk prote<strong>in</strong>, MW = 5.5 x 10 5 ). Because VLDLy conta<strong>in</strong>s only one<br />
apoB prote<strong>in</strong> per particle, this s<strong>in</strong>gle egg yolk would conta<strong>in</strong> 1.5 x 10 18 VLDLy
particles. The hen would be produc<strong>in</strong>g VLDLy particles at a rate <strong>of</strong> 1.5 x 10 14 particles<br />
per m<strong>in</strong>ute for seven days!!<br />
Biochemistry <strong>of</strong> immune processes.<br />
http://www.youtube.com/watch?v=Ys_V6FcYD5I&feature=related<br />
Viruses, bacteria, fungi, <strong>and</strong> parasites that enter the body <strong>of</strong> vertebrates <strong>of</strong> are<br />
recognized <strong>and</strong> attacked by the immune system. Endogenous cells that have<br />
undergone alterations— e. g., tumor cells—are also usually recognized as foreign<br />
<strong>and</strong> destroyed. The immune system is supported by physiological changes <strong>in</strong><br />
<strong>in</strong>fected tissue, known as <strong>in</strong>flammation. This reaction makes it easier for the<br />
immune cells to reach the site <strong>of</strong> <strong>in</strong>fection. Two different systems are <strong>in</strong>volved <strong>in</strong> the<br />
immune response. The <strong>in</strong>nate immune system is based on receptors that can<br />
dist<strong>in</strong>guish between bacterial <strong>and</strong> viral surface structures or foreign prote<strong>in</strong>s (known<br />
as antigens) <strong>and</strong> those that are endogenous. With the help <strong>of</strong> these receptors,<br />
phagocytes b<strong>in</strong>d to the pathogens, absorb them by endocytosis, <strong>and</strong> break them<br />
down. The complement system (see p. 298) is also part <strong>of</strong> the <strong>in</strong>nate system. The<br />
acquired (adaptive) immune system is based on the ability <strong>of</strong> the lymphocytes to<br />
form highly specific antigen receptors ―on suspicion,‖ without ever hav<strong>in</strong>g met the<br />
correspond<strong>in</strong>g antigen. In humans, there are several billion different lymphocytes,<br />
each <strong>of</strong> which carries a different antigen receptor. If this type <strong>of</strong> receptor recognizes<br />
―its‖ cognate antigen, the lymphocyte carry<strong>in</strong>g it is activated <strong>and</strong> then plays its<br />
special role <strong>in</strong> the immune response. In addition, a dist<strong>in</strong>ction is made between<br />
cellular <strong>and</strong> humoral immune responses.<br />
The T lymphocytes (T cells) are responsible for cellular immunity. They are<br />
named after the thymus, <strong>in</strong> which the decisive steps <strong>in</strong> their differentiation take<br />
place. Depend<strong>in</strong>g on their function, another dist<strong>in</strong>ction is made between cytotoxic T<br />
cells (green) <strong>and</strong> helper T cells (blue).
http://www.youtube.com/watch?v=14koX2tbRzU&feature=related<br />
http://www.youtube.com/watch?v=VOD5tuQ5wvo&feature=related<br />
Humoral immunity is based on the activity <strong>of</strong> the B lymphocytes (B cells, light<br />
brown), which mature <strong>in</strong> the bone marrow. After activation by T cells, B cells are able<br />
to release soluble forms <strong>of</strong> their specific antigen receptors, known as antibodies (see p.<br />
300), <strong>in</strong>to the <strong>blood</strong> plasma. The immune system’s ―memory‖ is represented by<br />
memory cells. These are particularly long–lived cells that can arise from any <strong>of</strong> the<br />
lymphocyte types described. Simplified diagram <strong>of</strong> the immune response.<br />
Pathogens that have entered the body—e. g., viruses (top)—are taken up by<br />
antigen-present<strong>in</strong>g cells (APCs) <strong>and</strong> proteolytically degraded (1). The viral fragments<br />
produced <strong>in</strong> this way are then presented on the surfaces <strong>of</strong> these cells with the help <strong>of</strong><br />
special membrane prote<strong>in</strong>s (MHC prote<strong>in</strong>s; see p. 296) (2). The APCs <strong>in</strong>clude B<br />
lymphocytes, macrophages, <strong>and</strong> dendritic cells such as the sk<strong>in</strong>’s Langerhans cells. The<br />
complexes <strong>of</strong> MHC prote<strong>in</strong>s <strong>and</strong> viral fragments displayed on the APCs are recognized<br />
by T cells that carry a receptor that matches the antigen (―T-cell receptors‖) (3).<br />
B<strong>in</strong>d<strong>in</strong>g leads to activation <strong>of</strong> the T cell concerned <strong>and</strong> selective replication <strong>of</strong> it (4,<br />
―clonal selection‖).
The proliferation <strong>of</strong> immune cells is stimulated by <strong>in</strong>terleuk<strong>in</strong>s (IL). These are a<br />
group <strong>of</strong> more than 20 signal<strong>in</strong>g substances belong<strong>in</strong>g to the cytok<strong>in</strong>e family (see p.<br />
392), with the help <strong>of</strong> which immune cells communicate with each other. For example,<br />
activated macrophages release IL-1 (5), while T cells stimulate their own replication<br />
<strong>and</strong> that <strong>of</strong> other immune cells by releas<strong>in</strong>g IL-2 (6). Depend<strong>in</strong>g on their type, activated<br />
T cells have different functions. Cytotoxic T cells (green) are able to recognize <strong>and</strong><br />
b<strong>in</strong>d virus<strong>in</strong>fected body cells or tumor cells (7). They then drive the <strong>in</strong>fected cells <strong>in</strong>to<br />
apoptosis (see p. 396) or kill them with perfor<strong>in</strong>, a prote<strong>in</strong> that perforates the target<br />
cell’s plasma membrane (8). B lymphocytes, which as APCs present viral fragments on
their surfaces, are recognized by helper T cells (blue) or their T cell receptors (9).<br />
Stimulated by <strong>in</strong>terleuk<strong>in</strong>s, selective clonal replication then takes place <strong>of</strong> B cells that<br />
carry antigen receptors match<strong>in</strong>g those <strong>of</strong> the pathogen (10). Thesemature <strong>in</strong>to plasma<br />
cells (11) <strong>and</strong> f<strong>in</strong>ally secrete large amounts <strong>of</strong> soluble antibodies (12).<br />
• Antigen receptors<br />
Many antigen receptors belong to the immunoglobul<strong>in</strong> superfamily. The<br />
common characteristic <strong>of</strong> these prote<strong>in</strong>s is that they aremade up from ―immunoglobul<strong>in</strong><br />
doma<strong>in</strong>s.‖ These are characteristically folded substructures consist<strong>in</strong>g <strong>of</strong> 70–110 am<strong>in</strong>o<br />
acids, which are also found <strong>in</strong> soluble immunoglobul<strong>in</strong>s (Ig; see p. 300). The<br />
illustration shows schematically a few <strong>of</strong> the important prote<strong>in</strong>s <strong>in</strong> the Ig superfamily.<br />
They consist <strong>of</strong> constant regions (brown or green) <strong>and</strong> variable regions (orange).<br />
Homologous doma<strong>in</strong>s are shown <strong>in</strong> the same colors <strong>in</strong> each case. All <strong>of</strong> the receptors<br />
have transmembrane helices at the C term<strong>in</strong>us, which anchor them to the membranes.<br />
Intramolecular <strong>and</strong> <strong>in</strong>termolecular disulfide bonds are also usually found <strong>in</strong> prote<strong>in</strong>s<br />
belong<strong>in</strong>g to the Ig family. Immunoglobul<strong>in</strong> M (IgM), a membrane prote<strong>in</strong> on the<br />
surface <strong>of</strong> B lymphocytes, serves to b<strong>in</strong>d free antigens to the B cells. By contrast, T cell<br />
receptors only b<strong>in</strong>d antigens when they are presented by another cell as a complex with<br />
an MHC prote<strong>in</strong> (see below). Interaction between MHC-bound antigens <strong>and</strong> T cell<br />
receptors is supported by co-receptors. This group <strong>in</strong>cludes CD8, a membrane prote<strong>in</strong><br />
that is typical <strong>in</strong> cytotoxic T cells. T helper cells use CD4 as a co-receptor <strong>in</strong>stead (not<br />
shown). The abbreviation ―CD‖ st<strong>and</strong>s for ―cluster <strong>of</strong> differentiation.‖ It is the term for<br />
a large group <strong>of</strong> prote<strong>in</strong>s that are all located on the cell surface <strong>and</strong> can therefore be<br />
identified by antibodies. In addition to CD4 <strong>and</strong> CD8, there are many other co-receptors<br />
on immune cells<br />
The MHC prote<strong>in</strong>s are named after the ―major histocompatibility complex‖—<br />
the DNA segment that codes for them. Human MHC prote<strong>in</strong>s are also known as<br />
HLA antigens (―human leukocyte-associated‖ antigens). Their polymorphism is so<br />
large that it is unlikely that any two <strong>in</strong>dividuals carry the same set <strong>of</strong> MHC<br />
prote<strong>in</strong>s—except formonozygotic tw<strong>in</strong>s. Class I MHC prote<strong>in</strong>s occur <strong>in</strong> almost all
nucleated cells. They ma<strong>in</strong>ly <strong>in</strong>teract with cytotoxic T cells <strong>and</strong> are the reason for<br />
the rejection <strong>of</strong> transplanted organs. Class I MHC prote<strong>in</strong>s are heterodimers (áâ).<br />
The â subunit is also known as â2-microglobul<strong>in</strong>. Class II MHC prote<strong>in</strong>s also<br />
consist <strong>of</strong> two peptide cha<strong>in</strong>s, which are related to each other. MHC II molecules are<br />
found on all antigen- present<strong>in</strong>g cells <strong>in</strong> the immune system. They serve for<br />
<strong>in</strong>teraction<br />
T-cell activation The illustration shows an <strong>in</strong>teraction between a virus-<strong>in</strong>fected<br />
body cell (bottom) <strong>and</strong> a CD8- carry<strong>in</strong>g cytotoxic T lymphocyte (top). The <strong>in</strong>fected cell<br />
breaks down viral prote<strong>in</strong>s <strong>in</strong> its cytoplasm (1) <strong>and</strong> transports the peptide fragments <strong>in</strong>to<br />
the endoplasmic reticulum with the help <strong>of</strong> a special transporter (TAP) (2). Newly<br />
synthesized class I MHC prote<strong>in</strong>s on the endoplasmic reticulum are loaded with one <strong>of</strong><br />
the peptides (3) <strong>and</strong> then transferred to the cell surface by vesicular transport (4). The<br />
viral peptides are bound on the surface <strong>of</strong> the á2 doma<strong>in</strong> <strong>of</strong> the MHC prote<strong>in</strong> <strong>in</strong> a<br />
depression formed by an <strong>in</strong>sertion as a ―floor‖ <strong>and</strong> two helices as ―walls‖ (see smaller<br />
illustration). Supported by CD8 <strong>and</strong> other co-receptors, a T cell with a match<strong>in</strong>g T cell
eceptor b<strong>in</strong>ds to the MHC peptide complex (5). This b<strong>in</strong>d<strong>in</strong>g activates prote<strong>in</strong> k<strong>in</strong>ases<br />
<strong>in</strong> the <strong>in</strong>terior <strong>of</strong> the T cell, which trigger a cha<strong>in</strong> <strong>of</strong> additional reactions (signal<br />
transduction). F<strong>in</strong>ally, destruction <strong>of</strong> the virus-<strong>in</strong>fected cell by the cytotoxic T<br />
lymphocytes takes place.<br />
Complement system<br />
The complement system is part <strong>of</strong> the <strong>in</strong>nate immune system (see p. 294). It<br />
supports nonspecific defense aga<strong>in</strong>st microorganisms. The system consists <strong>of</strong> some 30<br />
different prote<strong>in</strong>s, the ―complement factors,‖ which are found <strong>in</strong> the <strong>blood</strong> <strong>and</strong> represent<br />
about 4% <strong>of</strong> all plasma prote<strong>in</strong>s there. When <strong>in</strong>flammatory reactions occur, the<br />
complement factors enter the <strong>in</strong>fected tissue <strong>and</strong> take effect there. The complement<br />
system works <strong>in</strong> three different ways: Chemotaxis. Various complement factors attract<br />
immune cells that can attack <strong>and</strong> phagocytose pathogens. Opsonization. Certa<strong>in</strong><br />
complement factors (―opson<strong>in</strong>s‖) b<strong>in</strong>d to the pathogens <strong>and</strong> thereby mark them as<br />
targets for phagocytos<strong>in</strong>g cells (e. g., macrophages). Membrane attack. Other<br />
complement factors are deposited <strong>in</strong> the bacterial membrane, where they create pores<br />
that lyse the pathogen (see below).
• The reactions that take place <strong>in</strong> the complement system can be <strong>in</strong>itiated <strong>in</strong><br />
several ways. Dur<strong>in</strong>g the early phase <strong>of</strong> <strong>in</strong>fection, lipopolysaccharides <strong>and</strong> other<br />
structures on the surface <strong>of</strong> the pathogens trigger the alternative pathway (right).<br />
If antibodies aga<strong>in</strong>st the pathogens become available later, the antigen– antibody<br />
complexes formed activate the classic pathway (left). Acute-phase prote<strong>in</strong>s are<br />
also able to start the complement cascade (lect<strong>in</strong> pathway). Factors C1 to C4<br />
(for ―complement‖) belong to the classic pathway, while factors B <strong>and</strong> D form<br />
the reactive <strong>components</strong> <strong>of</strong> the alternative pathway. Factors C5 to C9 are<br />
responsible for membrane attack. Other <strong>components</strong> not shown here regulate the<br />
system. As <strong>in</strong> <strong>blood</strong> coagulation (see p. 290), the early <strong>components</strong> <strong>in</strong> the<br />
complement system are ser<strong>in</strong>e prote<strong>in</strong>ases, which mutually activate each other<br />
through limited proteolysis. They create a self-re<strong>in</strong>forc<strong>in</strong>g enzyme cascade.
Factor C3, the products <strong>of</strong> which are <strong>in</strong>volved <strong>in</strong> several functions, is central to the<br />
complement system. The classic pathway is triggered by the formation <strong>of</strong> factor C1 at<br />
IgG or IgM on the surface <strong>of</strong> microorganisms (left). C1 is an 18-part molecular<br />
complex with three different <strong>components</strong> (C1q, C1r, <strong>and</strong> C1s). C1q is shaped like a<br />
bunch <strong>of</strong> tulips, the ―flowers‖ <strong>of</strong> which b<strong>in</strong>d to the Fc region <strong>of</strong> antibodies (left). This<br />
activates C1r, a ser<strong>in</strong>e prote<strong>in</strong>ase that <strong>in</strong>itiates the cascade <strong>of</strong> the classic pathway. First,<br />
C4 is proteolytically activated <strong>in</strong>to C4b, which <strong>in</strong> turn cleaves C2 <strong>in</strong>to C2a <strong>and</strong> C2b.<br />
C4B <strong>and</strong> C2a together form C3 convertase [1], which f<strong>in</strong>ally catalyzes the cleavage <strong>of</strong><br />
C3 <strong>in</strong>to C3a <strong>and</strong> C3b. Small amounts <strong>of</strong> C3b also arise from non-enzymatic hydrolysis<br />
<strong>of</strong> C3.<br />
The classic pathway is triggered by the formation <strong>of</strong> factor C1 at IgG or IgM on<br />
the surface <strong>of</strong> microorganisms (left). C1 is an 18-part molecular complex with three<br />
different <strong>components</strong> (C1q, C1r, <strong>and</strong> C1s). C1q is shaped like a bunch <strong>of</strong> tulips, the<br />
―flowers‖ <strong>of</strong> which b<strong>in</strong>d to the Fc region <strong>of</strong> antibodies (left). This activates C1r, a ser<strong>in</strong>e<br />
prote<strong>in</strong>ase that <strong>in</strong>itiates the cascade <strong>of</strong> the classic pathway. First, C4 is proteolytically<br />
activated <strong>in</strong>to C4b, which <strong>in</strong> turn cleaves C2 <strong>in</strong>to C2a <strong>and</strong> C2b. C4B <strong>and</strong> C2a together<br />
form C3 convertase [1], which f<strong>in</strong>ally catalyzes the cleavage <strong>of</strong> C3 <strong>in</strong>to C3a <strong>and</strong> C3b.<br />
Small amounts <strong>of</strong> C3b also arise from non-enzymatic hydrolysis <strong>of</strong> C3. The alternative<br />
pathway starts with the b<strong>in</strong>d<strong>in</strong>g <strong>of</strong> factors C3b <strong>and</strong> B to bacterial lipopolysaccharides<br />
(endotox<strong>in</strong>s). The formation <strong>of</strong> this complex allows cleavage <strong>of</strong> B by factor D, giv<strong>in</strong>g<br />
rise to a second form <strong>of</strong> C3 convertase (C3bBb). Proteolytic cleavage <strong>of</strong> factor C3<br />
provides two <strong>components</strong> with different effects. The reaction exposes a highly reactive
thioester group <strong>in</strong> C3b, which reacts with hydroxyl or am<strong>in</strong>o groups. This allows C3b to<br />
b<strong>in</strong>d covalently to molecules on the bacterial surface (opsonization, right). In addition,<br />
C3b <strong>in</strong>itiates a cha<strong>in</strong> <strong>of</strong> reactions lead<strong>in</strong>g to the formation <strong>of</strong> the membrane attack<br />
complex Together with C4a <strong>and</strong> C5a (see below), the smaller product C3a promotes the<br />
<strong>in</strong>flammatory reaction <strong>and</strong> has chemotactic effects. The ―late‖ factors C5 to C9 are<br />
responsible for the development <strong>of</strong> the membrane attack complex (bottom). They<br />
create an ion-permeable pore <strong>in</strong> the bacterial membrane, which leads to lysis <strong>of</strong> the<br />
pathogen. This reaction is triggered by C5 convertase [2]. Depend<strong>in</strong>g on the type <strong>of</strong><br />
complement activation, this enzyme has the structure C4b2a3b or C3bBb3b, <strong>and</strong> it<br />
cleaves C5 <strong>in</strong>to C5a <strong>and</strong> C5b. The complex <strong>of</strong> C5b <strong>and</strong> C6 allows deposition <strong>of</strong> C7 <strong>in</strong><br />
the bacterial membrane. C8 <strong>and</strong> numerous C9 molecules—which form the actual<br />
pore—then b<strong>in</strong>d to this core. Antibodies<br />
http://www.youtube.com/watch?v=lrYlZJiuf18<br />
http://www.youtube.com/watch?v=Ys_V6FcYD5I&feature=related<br />
• Soluble antigen receptors, which are formed by activated B cells (plasma<br />
cells; see p. 294) <strong>and</strong> released <strong>in</strong>to the <strong>blood</strong>, are known as antibodies. They are
also members <strong>of</strong> the immunoglobul<strong>in</strong> family (Ig; see p. 296). Antibodies are an<br />
important part <strong>of</strong> the humoral immune defense system. They have no<br />
antimicrobial properties themselves, but support the cellular immune system <strong>in</strong><br />
various ways: 1. They b<strong>in</strong>d to antigens on the surface <strong>of</strong> pathogens <strong>and</strong> thereby<br />
prevent them from <strong>in</strong>teract<strong>in</strong>g with body cells (neutralization; see p. 404, for<br />
example). 2. They l<strong>in</strong>k s<strong>in</strong>gle-celled pathogens <strong>in</strong>to aggregates (immune<br />
complexes), which are more easily taken up by phagocytes (agglut<strong>in</strong>ation). 3.<br />
They activate the complement system (see p. 298) <strong>and</strong> thereby promote the <strong>in</strong>nate<br />
immune defense system (opsonization). In addition, antibodies have become<br />
<strong>in</strong>dispensable aids <strong>in</strong> medical <strong>and</strong> biological diagnosis. Doma<strong>in</strong> structure <strong>of</strong><br />
immunoglobul<strong>in</strong> G _<br />
Type G immunoglobul<strong>in</strong>s (IgG) are quantitatively the most important antibodies<br />
<strong>in</strong> the <strong>blood</strong>,where they form the fraction <strong>of</strong> ã-globul<strong>in</strong>s (see p. 276). IgGs (mass 150<br />
kDa) are tetramers with two heavy cha<strong>in</strong>s (H cha<strong>in</strong>s; red or orange) <strong>and</strong> two light<br />
cha<strong>in</strong>s (L cha<strong>in</strong>s; yellow). Both H cha<strong>in</strong>s are glycosylated (violet; see also p. 43). The<br />
prote<strong>in</strong>ase papa<strong>in</strong> cleaves IgG <strong>in</strong>to two Fab fragments <strong>and</strong> one Fc fragment. The Fab<br />
(―antigen-b<strong>in</strong>d<strong>in</strong>g‖) fragments, which each consist <strong>of</strong> one L cha<strong>in</strong> <strong>and</strong> the N-term<strong>in</strong>al<br />
part <strong>of</strong> an H cha<strong>in</strong>, are able to b<strong>in</strong>d antigens. The Fc (―crystallizable‖) fragment is made<br />
up <strong>of</strong> the C-term<strong>in</strong>al halves <strong>of</strong> the two H cha<strong>in</strong>s. This segment serves to b<strong>in</strong>d IgG to cell<br />
surfaces, for <strong>in</strong>teraction with the complement system <strong>and</strong> antibody transport.<br />
Immunoglobul<strong>in</strong>s are constructed <strong>in</strong> a modular fashion from several immunoglobul<strong>in</strong><br />
doma<strong>in</strong>s (shown <strong>in</strong> the diagram on the right <strong>in</strong> Ω form). Classes <strong>of</strong> immunoglobul<strong>in</strong>s<br />
_<br />
http://www.youtube.com/watch?v=mUXIK5gGD1k<br />
Human immunoglobul<strong>in</strong>s are divided <strong>in</strong>to five classes. IgA (with two subgroups),<br />
IgD, IgE, IgG (with four subgroups), <strong>and</strong> IgM are def<strong>in</strong>ed by their H cha<strong>in</strong>s, which are<br />
designated by the Greek letters á, ä, å, ã, <strong>and</strong> µ. By contrast, there are only two types <strong>of</strong>
L cha<strong>in</strong> (ê <strong>and</strong> ë). IgD <strong>and</strong> IgE (like IgG) are tetramers with the structure H2L2. By<br />
contrast, soluble IgA <strong>and</strong> IgM are multimers that are held together by disulfide bonds<br />
<strong>and</strong> additional J peptides (jo<strong>in</strong><strong>in</strong>g peptides). The antibodies have different tasks. IgMs<br />
are the first immunoglobul<strong>in</strong>s formed after contact with a foreign antigen. Their early<br />
forms are located on the surface <strong>of</strong> B cells (see p. 296), while the later forms are<br />
secreted from plasma cells as pentamers. Their action targets microorganisms <strong>in</strong><br />
particular. Quantitatively, IgGs are the most important immunoglobul<strong>in</strong>s (see the table<br />
show<strong>in</strong>g serum concentrations). They occur <strong>in</strong> the <strong>blood</strong> <strong>and</strong> <strong>in</strong>terstitial fluid. As they<br />
can pass the placenta with the help <strong>of</strong> receptors, they can be transferred from mother to<br />
fetus. IgAs ma<strong>in</strong>ly occur <strong>in</strong> the <strong>in</strong>test<strong>in</strong>al tract <strong>and</strong> <strong>in</strong> body secretions. IgEs are found <strong>in</strong><br />
low concentrations <strong>in</strong> the <strong>blood</strong>. As they can trigger degranulation <strong>of</strong> mast cells (see p.<br />
380), they play an important role <strong>in</strong> allergic reactions. The function <strong>of</strong> IgDs is still<br />
unexpla<strong>in</strong>ed. Their plasma concentration is also very low.<br />
Causes <strong>of</strong> antibody variety _
There are three reasons for the extremely wide variability <strong>of</strong> antibodies: 1.<br />
Multiple genes. Various genes are available to code for the variable prote<strong>in</strong> doma<strong>in</strong>s.<br />
Only one gene from among these is selected <strong>and</strong> expressed. 2. Somatic recomb<strong>in</strong>ation.<br />
The genes are divided <strong>in</strong>to several segments, <strong>of</strong> which there are various versions.<br />
Various (―untidy‖) comb<strong>in</strong>ations <strong>of</strong> the segments dur<strong>in</strong>g lymphocyte maturation give<br />
rise to r<strong>and</strong>omly comb<strong>in</strong>ed new genes (―mosaic genes‖). 3. Somaticmutation. Dur<strong>in</strong>g<br />
differentiation <strong>of</strong> B cells <strong>in</strong>to plasma cells, the cod<strong>in</strong>g genes mutate. In this way, the<br />
―primordial‖ germl<strong>in</strong>e genes can become different somatic genes <strong>in</strong> the <strong>in</strong>dividual B<br />
cell clones.<br />
Biosynthesis <strong>of</strong> a light cha<strong>in</strong> _<br />
We can look at the basic features <strong>of</strong> the genetic organization <strong>and</strong> synthesis <strong>of</strong><br />
immunoglobul<strong>in</strong>s us<strong>in</strong>g the biosynthesis <strong>of</strong> a mouse ê cha<strong>in</strong> as an example. The gene<br />
segments for this light cha<strong>in</strong> are designated L, V, J, <strong>and</strong> C. They are located on<br />
chromosome 6 <strong>in</strong> the germ-l<strong>in</strong>e DNA (on chromosome 2 <strong>in</strong> humans) <strong>and</strong> are separated<br />
from one another by <strong>in</strong>trons (see p. 242) <strong>of</strong> different lengths. Some 150 identical L
segments code for the signal peptide (―leader sequence,‖ 17–20 am<strong>in</strong>o acids) for<br />
secretion <strong>of</strong> the product<br />
The V segments, <strong>of</strong> which there are 150 different variants, code formost <strong>of</strong> the<br />
variable doma<strong>in</strong>s (95 <strong>of</strong> the 108 am<strong>in</strong>o acids). L <strong>and</strong> V segments always occur <strong>in</strong><br />
pairs—<strong>in</strong> t<strong>and</strong>em, so to speak. By contrast, there are only five variants <strong>of</strong> the J<br />
segments (jo<strong>in</strong><strong>in</strong>g segments) at most. These code for a peptide with 13 am<strong>in</strong>o acids that<br />
l<strong>in</strong>ks the variable part <strong>of</strong> the ê cha<strong>in</strong>s to the constant part. A s<strong>in</strong>gle C segment codes for<br />
the constant part <strong>of</strong> the light cha<strong>in</strong> (84 am<strong>in</strong>o acids). Dur<strong>in</strong>g the differentiation <strong>of</strong> B<br />
lymphocytes, <strong>in</strong>dividual V/J comb<strong>in</strong>ations arise <strong>in</strong> each B cell. One <strong>of</strong> the 150 L/V<br />
t<strong>and</strong>em segments is selected <strong>and</strong> l<strong>in</strong>ked to one <strong>of</strong> the five J segments.<br />
The immune system is a complex, dynamic, <strong>and</strong> beautifully orchestrated<br />
mechanism with enormous responsibility. It defends aga<strong>in</strong>st foreign <strong>in</strong>vasion by<br />
microorganisms, screens out cancer cells, adapts as we grow, <strong>and</strong> modifies how we<br />
<strong>in</strong>teract with our environment. When it malfunctions, disease, cancer or death can<br />
occur. Although it is not necessary to underst<strong>and</strong> all the <strong>in</strong>timate details <strong>of</strong> the immune<br />
system, it is wise to have a basic grasp <strong>of</strong> its functions. More precisely, we should<br />
underst<strong>and</strong> how to stay healthy.<br />
Tra<strong>in</strong><strong>in</strong>g the immune system -- the "J" curve<br />
It appears that the immune system has a tra<strong>in</strong><strong>in</strong>g effect, similar to other areas <strong>of</strong><br />
physiology (e.g., cardiovascular, muscular). In other words, a balanced tra<strong>in</strong><strong>in</strong>g<br />
program <strong>of</strong> exercise <strong>and</strong> rest leads to better performance. Studies <strong>in</strong> the laboratory <strong>and</strong><br />
epidemiological observations have shown improved immune function <strong>and</strong> fewer URIs<br />
<strong>in</strong> athletes as compared to their couch-potato counterparts. This is especially true <strong>in</strong><br />
older athletes <strong>and</strong> it appears that regular exercise can help attenuate the age related<br />
decl<strong>in</strong>e <strong>in</strong> immune function.<br />
On the other h<strong>and</strong>, too much exercise can lead to a dramatically <strong>in</strong>creased risk <strong>of</strong><br />
URIs. The stress <strong>of</strong> strenuous exercise transiently suppresses immune function. This<br />
<strong>in</strong>terruption <strong>of</strong> otherwise vigorous surveillance can provide an "open w<strong>in</strong>dow" for a
variety <strong>of</strong> <strong>in</strong>fectious diseases -- notably viral illnesses -- to take hold. This is especially<br />
true follow<strong>in</strong>g s<strong>in</strong>gle bouts <strong>of</strong> excessive exercise. For example, it has been observed<br />
that two-thirds <strong>of</strong> participants developed URIs shortly after complet<strong>in</strong>g an<br />
ultramarathon. Similarly, cumulative overtra<strong>in</strong><strong>in</strong>g weakens the athlete's immune<br />
system, lead<strong>in</strong>g to frequent illness <strong>and</strong> <strong>in</strong>jury.<br />
The best model that accommodates cl<strong>in</strong>ical observations <strong>and</strong> laboratory<br />
experiments is described by the "J"-curve ( Fig. 1). It is important to note that this curve<br />
is <strong>in</strong>dividualized. What is moderate tra<strong>in</strong><strong>in</strong>g for some is overtra<strong>in</strong><strong>in</strong>g for others.<br />
Stress is cumulative<br />
In addition to strenuous exercise, other forms <strong>of</strong> stress may also transiently<br />
suppress immune function. S<strong>in</strong>ce exercise is not the only stress factor, an athlete must<br />
consider a host <strong>of</strong> other variables. There are job responsibilities, family obligations,<br />
social <strong>in</strong>teractions, f<strong>in</strong>ancial concerns <strong>and</strong> other <strong>components</strong> that shape our lives. The<br />
sum <strong>of</strong> all <strong>of</strong> these affects a central axis <strong>in</strong> the body which ultimately <strong>in</strong>fluences<br />
immune function. Some <strong>of</strong> these (e.g., exercise) are under our direct control, <strong>and</strong> others<br />
only partially or not at all. Recogniz<strong>in</strong>g when excess stress occurs is easier if it just<br />
comes from one source. However, all too <strong>of</strong>ten it is the sum <strong>of</strong> many small, difficult to
ecognize changes that tips the scales <strong>and</strong> sends the athlete <strong>in</strong>to the whirlpool <strong>of</strong><br />
overtra<strong>in</strong><strong>in</strong>g <strong>and</strong> immunosuppression. Alone <strong>and</strong> <strong>in</strong> isolation these small changes would<br />
be manageable, but comb<strong>in</strong>ed they can overwhelm. (Fig. 2.)<br />
Recommendations<br />
Currently, the best way to stay healthy is to listen to your body. Recogniz<strong>in</strong>g the<br />
early warn<strong>in</strong>g signs <strong>and</strong> adapt<strong>in</strong>g the tra<strong>in</strong><strong>in</strong>g schedule accord<strong>in</strong>gly can help keep you<br />
healthy. In that light, here are some po<strong>in</strong>ts to ponder <strong>and</strong> a few recommendations,<br />
Keep a tra<strong>in</strong><strong>in</strong>g log. In addition to record<strong>in</strong>g workouts, keep a fatigue score<br />
(scale 0-5). It is expected that a hard workout will make you tired, so it is more<br />
important to note the cumulative "feel" dur<strong>in</strong>g the day. Granted, the scale is<br />
<strong>in</strong>dividualized <strong>and</strong> subjective, but this simple tool is very useful. If you notice<br />
that your fatigue is progressively <strong>in</strong>creas<strong>in</strong>g over days or weeks, then it is time to<br />
add more rest.<br />
A properly constructed tra<strong>in</strong><strong>in</strong>g program that allows for rest <strong>and</strong> recovery<br />
will help head <strong>of</strong>f problems before they start. Periodization is a way to achieve<br />
that goal.<br />
Record your rest<strong>in</strong>g morn<strong>in</strong>g heart rate. A progressive <strong>in</strong>crease may tip you<br />
<strong>of</strong>f that you are exceed<strong>in</strong>g your ability to recover.
Anticipate added stress <strong>in</strong> advance (e.g. new job) <strong>and</strong> adjust the workout<br />
schedule correspond<strong>in</strong>gly. A small amount <strong>of</strong> rest early will prevent a bigger<br />
problem later.<br />
To make sure your anti-oxidant defense system is tuned up, eat five<br />
serv<strong>in</strong>gs <strong>of</strong> fruit or vegetables per day. Note: vitam<strong>in</strong> supplements do not appear<br />
to have the same benefits as fruits <strong>and</strong> vegetables.<br />
October)<br />
Heed your body's early warn<strong>in</strong>g signs,<br />
o Disordered sleep (too much or <strong>in</strong>somnia)<br />
o Loss <strong>of</strong> <strong>in</strong>terest <strong>in</strong> pleasurable activities<br />
o Mood<strong>in</strong>ess or depression<br />
o Excessive muscle soreness<br />
o Poor concentration. Lack <strong>of</strong> mental energy.<br />
o Altered appetite.<br />
o Frequent <strong>in</strong>jury or illness<br />
o Lack <strong>of</strong> physical energy<br />
Get an annual <strong>in</strong>fluenza vacc<strong>in</strong>e (usually available each year start<strong>in</strong>g <strong>in</strong><br />
Because frequent URIs or unrelent<strong>in</strong>g fatigue may be a sign <strong>of</strong> an<br />
underly<strong>in</strong>g illness, it is recommended that you consult your physician.<br />
The Anatomy <strong>of</strong> the Immune System<br />
The organs <strong>of</strong> the immune system are stationed throughout the body. They are<br />
generally referred to as lymphoid organs because they are concerned with the growth,<br />
development, <strong>and</strong> deployment <strong>of</strong> lymphocytes, the white cells that are the key<br />
operatives <strong>of</strong> the immune system. Lymphoid organs <strong>in</strong>clude the bone marrow <strong>and</strong> the<br />
thymus, as well as lymph nodes, spleen, tonsils <strong>and</strong> adenoids, the appendix, <strong>and</strong> clumps<br />
<strong>of</strong> lymphoid tissue <strong>in</strong> the small <strong>in</strong>test<strong>in</strong>e known as Peyer's patches. The <strong>blood</strong> <strong>and</strong><br />
lymphatic vessels that carry lymphocytes to <strong>and</strong> from the other structures can also be<br />
considered lymphoid organs.
Cells dest<strong>in</strong>ed to become immune cells, like all other <strong>blood</strong> cells, are produced <strong>in</strong><br />
the bone marrow, the s<strong>of</strong>t tissue <strong>in</strong> the hollow shafts <strong>of</strong> long bones. The descendants <strong>of</strong><br />
some so-called stem cells become lymphocytes, while others develop <strong>in</strong>to a second<br />
major group <strong>of</strong> immune cells typified by the large, cell-<strong>and</strong> particle-devour<strong>in</strong>g white<br />
cells known as phagocytes.<br />
The two major classes <strong>of</strong> lymphocytes are B cells <strong>and</strong> T cells. B cells complete<br />
their maturation <strong>in</strong> the bone marrow. T cells, on the other h<strong>and</strong>, migrate to the thymus,<br />
a multilobed organ that lies high beh<strong>in</strong>d the breastbone. There they multiply <strong>and</strong> mature<br />
<strong>in</strong>to cells capable <strong>of</strong> produc<strong>in</strong>g immune response-that is, they become<br />
immunocompetent. In a process referred to as T cell "education," T cells <strong>in</strong> the thymus<br />
learn to dist<strong>in</strong>guish self cells from nonself cells; T cells that would react aga<strong>in</strong>st self<br />
antigens are elim<strong>in</strong>ated.
Upon exit<strong>in</strong>g the bone marrow <strong>and</strong> thymus, some lymphocytes congregate <strong>in</strong><br />
immune organs or lymph nodes. Others-both B <strong>and</strong> T cells-travel widely <strong>and</strong><br />
cont<strong>in</strong>uously throughout the body. They use the <strong>blood</strong> circulation as well as a bodywide<br />
network <strong>of</strong> lymphatic vessels similar to <strong>blood</strong> vessels.<br />
Laced along the lymphatic routes-with clusters <strong>in</strong> the neck, armpits, abdomen, <strong>and</strong><br />
gro<strong>in</strong>-are small, bean-shaped lymph nodes. Each lymph node conta<strong>in</strong>s specialized<br />
compartments that house platoons <strong>of</strong> B lymphocytes, T lymphocytes, <strong>and</strong> other cells<br />
capable <strong>of</strong> enmesh<strong>in</strong>g antigen <strong>and</strong> present<strong>in</strong>g it to T cells. Thus, the lymph node br<strong>in</strong>gs<br />
together the several <strong>components</strong> needed to spark an immune response.<br />
The spleen, too, provides a meet<strong>in</strong>g ground for immune defenses. A fist-sized<br />
organ at the upper left <strong>of</strong> the abdomen, the spleen conta<strong>in</strong>s two ma<strong>in</strong> types <strong>of</strong> tissue: the<br />
red pulp that disposes <strong>of</strong> worn-out <strong>blood</strong> cells <strong>and</strong> the white pulp that conta<strong>in</strong>s<br />
lymphoid tissue. Like the lymph nodes, the spleen's lymphoid tissue is subdivided <strong>in</strong>to<br />
compartments that specialize <strong>in</strong> different k<strong>in</strong>ds <strong>of</strong> immune cells. Microorganisms<br />
carried by the <strong>blood</strong> <strong>in</strong>to the red pulp become trapped by the immune cells known as<br />
macrophages. (Although people can live without a spleen, persons whose spleens have<br />
been damaged by trauma or by disease such as sickle cell anemia, are highly susceptible
to <strong>in</strong>fection; surgical removal <strong>of</strong> the spleen is especially dangerous for young children<br />
<strong>and</strong> the immunosuppressed.)<br />
Nonencapsulated clusters <strong>of</strong> lymphoid tissue are found <strong>in</strong> many parts <strong>of</strong> the body.<br />
They are common around the mucous membranes l<strong>in</strong><strong>in</strong>g the respiratory <strong>and</strong> digestive<br />
tracts-areas that serve as gateways to the body. They <strong>in</strong>clude the tonsils <strong>and</strong> adenoids,<br />
the appendix, <strong>and</strong> Peyer's patches.<br />
The lymphatic vessels carry lymph, a clear fluid that bathes the body's tissues.<br />
Lymph, along with the many cells <strong>and</strong> particles it carries-notably lymphocytes,<br />
macrophages, <strong>and</strong> foreign antigens, dra<strong>in</strong>s out <strong>of</strong> tissues <strong>and</strong> seeps across the th<strong>in</strong> walls<br />
<strong>of</strong> t<strong>in</strong>y lymphatic vessels. The vessels transport the mix to lymph nodes, where antigens<br />
can be filtered out <strong>and</strong> presented to immune cells.<br />
Additional lymphocytes reach the lymph nodes (<strong>and</strong> other immune tissues)<br />
through the <strong>blood</strong>stream. Each node is supplied by an artery <strong>and</strong> a ve<strong>in</strong>; lymphocytes<br />
enter the node by travers<strong>in</strong>g the walls <strong>of</strong> the very small specialized ve<strong>in</strong>s.<br />
All lymphocytes exit lymph nodes <strong>in</strong> lymph via outgo<strong>in</strong>g lymphatic vessels. Much<br />
as small creeks <strong>and</strong> streams empty <strong>in</strong>to larger rivers, the lymphatics feed <strong>in</strong>to larger <strong>and</strong><br />
larger channels. At the base <strong>of</strong> the neck, large lymphatic vessels merge <strong>in</strong>to the thoracic<br />
duct, which empties its contents <strong>in</strong>to the <strong>blood</strong>stream.
Once <strong>in</strong> the <strong>blood</strong>stream, the lymphocytes <strong>and</strong> other assorted immune cells are<br />
transported to tissues throughout the body. They patrol everywhere for foreign antigens,<br />
then gradually drift back <strong>in</strong>to the lymphatic vessels, to beg<strong>in</strong> the cycle all over aga<strong>in</strong><br />
Disorders <strong>of</strong> the Immune System: Allergy<br />
http://www.youtube.com/watch?v=NFTL51FvX4Q&feature=related<br />
The most common types <strong>of</strong> allergic reactions-hay fever, some k<strong>in</strong>ds <strong>of</strong> asthma, <strong>and</strong><br />
hives-are produced when the immune system response to a false alarm. In a susceptible<br />
person, a <strong>normal</strong>ly harmless substance-grass pollen or house dust, for example-is<br />
perceived as a threat <strong>and</strong> is attacked.<br />
Such allergic reactions are related to the antibody known as immunoglobul<strong>in</strong> E.<br />
Like other antibodies, each IgE antibody is specific; one reacts aga<strong>in</strong>st oak pollen,<br />
another aga<strong>in</strong>st ragweed. The role <strong>of</strong> IgE <strong>in</strong> the natural order is not known, although<br />
some scientists suspect that it developed as a defense aga<strong>in</strong>st <strong>in</strong>fection by parasitic<br />
worms.
The first time an allergy-prone person is exposed to an allergen, he or she makes<br />
large amounts <strong>of</strong> the correspond<strong>in</strong>g IgE antibody. These IgE molecules attach to the<br />
surfaces <strong>of</strong> mast cells (<strong>in</strong> tissue) or basophils (<strong>in</strong> the circulation). Mast cells are plentiful<br />
<strong>in</strong> the lungs, sk<strong>in</strong>, tongue, <strong>and</strong> l<strong>in</strong><strong>in</strong>gs <strong>of</strong> the nose <strong>and</strong> <strong>in</strong>test<strong>in</strong>al tract.<br />
When an IgE antibody sit<strong>in</strong>g on a mast cell or basophil encounters its specific<br />
allergen, the IgE antibody signals the mast cell or basophil to release the powerful<br />
chemicals stored with<strong>in</strong> its granules. These chemicals <strong>in</strong>clude histam<strong>in</strong>e, hepar<strong>in</strong>, <strong>and</strong><br />
substances that activate <strong>blood</strong> platelets <strong>and</strong> attract secondary cells such as eos<strong>in</strong>ophils<br />
<strong>and</strong> neutrophils. The activated mast cell or basophil also synthesizes new mediators,<br />
<strong>in</strong>clud<strong>in</strong>g prostagl<strong>and</strong><strong>in</strong>s <strong>and</strong> leukotrienes, on the spot.
It is such chemical mediators that cause the symptoms <strong>of</strong> allergy, <strong>in</strong>clud<strong>in</strong>g<br />
wheez<strong>in</strong>g, sneez<strong>in</strong>g, runny eyes <strong>and</strong> itch<strong>in</strong>g. They can also produce anaphylactic shock,<br />
a life-threaten<strong>in</strong>g allergic reaction characterized by swell<strong>in</strong>g <strong>of</strong> body tissues, <strong>in</strong>clud<strong>in</strong>g<br />
the throat, <strong>and</strong> a sudden fall <strong>in</strong> <strong>blood</strong> pressure.<br />
Autoimmune Diseases<br />
Sometimes the immune system's recognition apparatus breaks down, <strong>and</strong> the body<br />
beg<strong>in</strong>s to manufacture antibodies <strong>and</strong> T cells directed aga<strong>in</strong>st the body's own<br />
constituents-cells, cell <strong>components</strong>, or specific organs. Such antibodies are known as<br />
autoantibodies, <strong>and</strong> the diseases they produce are called autoimmune diseases. (Not all<br />
autoantibodies are harmful; some types appear to be <strong>in</strong>tegral to the immune system's<br />
regulatory scheme.)<br />
Autoimmune reactions contribute to many enigmatic diseases. For <strong>in</strong>stance,<br />
autoantibodies to red <strong>blood</strong> cells can cause anemia, autoantibodies to pancreas cells<br />
contribute to juvenile diabetes, <strong>and</strong> autoantibodies to nerve <strong>and</strong> muscle cells are found<br />
<strong>in</strong> patients with the chronic muscle weakness known as myasthenia gravis.<br />
Autoantibody known as rheumatoid factor is common <strong>in</strong> persons with rheumatoid<br />
arthritis.<br />
Persons with systemic lupus erythematosus (SLE), whose symptoms encompass<br />
many systems, have antibodies to many types <strong>of</strong> cells <strong>and</strong> cellular <strong>components</strong>. These<br />
<strong>in</strong>clude antibodies directed aga<strong>in</strong>st substances found <strong>in</strong> the cell's nucleus-DNA, RNA,<br />
or prote<strong>in</strong>s-which are known as ant<strong>in</strong>uclear antibodies, or ANAs. These antibodies can<br />
cause serious damage when they l<strong>in</strong>k up with self antigens to form circulat<strong>in</strong>g immune
complexes, which become lodged <strong>in</strong> body tissue <strong>and</strong> set <strong>of</strong>f <strong>in</strong>flammatory reactions<br />
(Immune Complex Diseases).<br />
Autoimmune diseases affect the immune system at several levels. In patients with<br />
SLE, for <strong>in</strong>stance, B cells are hyperactive while suppressor cells are underactive; it is<br />
not clear which defect comes first. Moreover, production <strong>of</strong> IL-2 is low, while levels <strong>of</strong><br />
gamma <strong>in</strong>terferon are high. Patients with rheumatoid arthritis, who have a defective<br />
suppressor T cell system, cont<strong>in</strong>ue to make antibodies to a common virus, whereas the<br />
response <strong>normal</strong>ly shuts down after about a dozen days.<br />
No one knows just what causes an autoimmune disease, but several factors are<br />
likely to be <strong>in</strong>volved. These may <strong>in</strong>clude viruses <strong>and</strong> environmental factors such as<br />
exposure to sunlight, certa<strong>in</strong> chemicals, <strong>and</strong> some drugs, all <strong>of</strong> which may damage or<br />
alter body cells so that they are no longer recognizable as self. Sex hormones may be<br />
important, too, s<strong>in</strong>ce most autoimmune diseases are far more common <strong>in</strong> women than <strong>in</strong><br />
men.<br />
Heredity also appears to play a role. Autoimmune reactions, like many other<br />
immune responses, are <strong>in</strong>fluenced by the genes <strong>of</strong> the MHC. A high proportion <strong>of</strong><br />
human patients with autoimmune disease have particular histocompatibility types. For<br />
example, many persons with rheumatoid arthritis display the self marker known as<br />
HLA-DR4.<br />
Many types <strong>of</strong> therapies are be<strong>in</strong>g used to combat autoimmune diseases. These<br />
<strong>in</strong>clude corticosteroids, immunosuppressive drugs developed as anticancer agents,<br />
radiation <strong>of</strong> the lymph nodes, <strong>and</strong> plasmapheresis, a sort <strong>of</strong> "<strong>blood</strong> wash<strong>in</strong>g" that<br />
removes diseased cells <strong>and</strong> harmful molecules from the circulation.<br />
Immune Complex Diseases
Immune complexes are clusters <strong>of</strong> <strong>in</strong>terlock<strong>in</strong>g antigens <strong>and</strong> antibodies. Under<br />
<strong>normal</strong> conditions immune complexes are rapidly removed from the <strong>blood</strong>stream by<br />
macrophages <strong>in</strong> the spleen <strong>and</strong> Kupffer cells <strong>in</strong> the liver. In some circumstances,<br />
however, immune complexes cont<strong>in</strong>ue to circulate. Eventually they become trapped <strong>in</strong><br />
the tissues <strong>of</strong> the kidneys, lung, sk<strong>in</strong>, jo<strong>in</strong>ts, or <strong>blood</strong> vessels. Just where they end up<br />
probably depends on the nature <strong>of</strong> the antigen, the class <strong>of</strong> antibody-IgG, for <strong>in</strong>stance,<br />
<strong>in</strong>stead <strong>of</strong> IgM-<strong>and</strong> the size <strong>of</strong> the complex. There they set <strong>of</strong>f reactions that lead to<br />
<strong>in</strong>flammation <strong>and</strong> tissue damage.<br />
Immune complexes work their damage <strong>in</strong> many diseases. Sometimes, as is the case<br />
with malaria <strong>and</strong> viral hepatitis, they reflect persistent low-grade <strong>in</strong>fections. Sometimes<br />
they arise <strong>in</strong> response to environmental antigens such as the moldy hay that causes the<br />
disease known as farmer's lung. Frequently, immune complexes develop <strong>in</strong> autoimmune<br />
disease, where the cont<strong>in</strong>uous production <strong>of</strong> autoantibodies overloads the immune<br />
complex removal system.<br />
Immunodeficiency Diseases<br />
Lack <strong>of</strong> one or more <strong>components</strong> <strong>of</strong> the immune system results <strong>in</strong><br />
immunodeficiency disorders. These can be <strong>in</strong>herited, acquired through <strong>in</strong>fection or<br />
other illness, or produced as an <strong>in</strong>advertent side effect <strong>of</strong> certa<strong>in</strong> drug treatments.<br />
People with advanced cancer may experience immune deficiencies as a result <strong>of</strong><br />
the disease process or from extensive anticancer therapy. Transient immune
deficiencies can develop <strong>in</strong> the wake <strong>of</strong> common viral <strong>in</strong>fections, <strong>in</strong>clud<strong>in</strong>g <strong>in</strong>fluenza,<br />
<strong>in</strong>fectious mononucleosis, <strong>and</strong> measles. Immune responsiveness can also be depressed<br />
by <strong>blood</strong> transfusions, surgery malnutrition, <strong>and</strong> stress.<br />
Some children are born with defects <strong>in</strong> their immune systems. Those with flaws <strong>in</strong><br />
the B cell <strong>components</strong> are unable to produce antibodies (immunoglobul<strong>in</strong>s). These<br />
conditions, known as agammaglobul<strong>in</strong>emias or hypogammaglobul<strong>in</strong>emias, leave the<br />
children vulnerable to <strong>in</strong>fectious organisms; such disorders can be combated with<br />
<strong>in</strong>jections <strong>of</strong> immunoglobul<strong>in</strong>s.<br />
Other children, whose thymus is either miss<strong>in</strong>g or small <strong>and</strong> ab<strong>normal</strong>, lack T<br />
cells. The resultant disorders have been treated with thymic transplants.<br />
Very rarely, <strong>in</strong>fants are born lack<strong>in</strong>g all the major immune defenses; this is known<br />
as severe comb<strong>in</strong>ed immunodeficiency disease (SCID). Some children with SCID have<br />
lived for years <strong>in</strong> germ-free rooms <strong>and</strong> "bubbles." A few SCID patients have been<br />
successfully treated with transplants <strong>of</strong> bone marrow (Bone Marrow Transplants).<br />
The devastat<strong>in</strong>g immunodeficiency disorder known as the acquired<br />
immunodeficiency syndrome (AIDS) was first recognized <strong>in</strong> 1981. Caused by a virus<br />
(the human immunodeficiency virus, or HIV) that destroys T4 cells <strong>and</strong> that is harbored<br />
<strong>in</strong> macrophages as well as T4 cells, AIDS is characterized by a variety <strong>of</strong> unusual<br />
<strong>in</strong>fections <strong>and</strong> otherwise rare cancers. The AIDS virus also damages tissue <strong>of</strong> the bra<strong>in</strong><br />
<strong>and</strong> sp<strong>in</strong>al cord, produc<strong>in</strong>g progressive dementia.
AIDS <strong>in</strong>fections are known as "opportunistic" because they are produced by<br />
commonplace organisms that do not trouble people whose immune systems are healthy,<br />
but which take advantage <strong>of</strong> the "opportunity" provided by an immune defense <strong>in</strong><br />
disarray. The most common <strong>in</strong>fection is an unusual <strong>and</strong> life-threaten<strong>in</strong>g form <strong>of</strong><br />
pneumonia caused by a one-celled organism (a Protozoa) called Pneumocystis car<strong>in</strong>ii.<br />
AIDS patients are also susceptible to unusual lymphomas <strong>and</strong> Kaposi's sarcoma, a rare<br />
cancer that results from the ab<strong>normal</strong> proliferation <strong>of</strong> endothelial cells <strong>in</strong> the <strong>blood</strong><br />
vessels.<br />
Some persons <strong>in</strong>fected with the AIDS virus develop a condition known as AIDS-<br />
related complex, or ARC, characterized by fatigue, fever, weight loss, diarrhea, <strong>and</strong><br />
swollen lymph gl<strong>and</strong>s. Yet other persons who are <strong>in</strong>fected with the AIDS virus<br />
apparently rema<strong>in</strong> well; however, even though they develop no symptoms, they can<br />
transmit the virus to others.<br />
AIDS is a contagious disease, spread by <strong>in</strong>timate sexual contact, by direct<br />
<strong>in</strong>oculation <strong>of</strong> the virus <strong>in</strong>to the <strong>blood</strong>stream, or from mother to child dur<strong>in</strong>g pregnancy.<br />
Most <strong>of</strong> the AIDS cases <strong>in</strong> the United States have been found among homosexual <strong>and</strong><br />
bisexual men with multiple sex partners, <strong>and</strong> among <strong>in</strong>travenous drug abusers. Others<br />
have <strong>in</strong>volved men who received untreated <strong>blood</strong> products for hemophilia; persons who<br />
received transfusions <strong>of</strong> <strong>in</strong>advertently contam<strong>in</strong>ated <strong>blood</strong>-primarily before the AIDS<br />
virus was discovered <strong>and</strong> virtually elim<strong>in</strong>ated from the nation's <strong>blood</strong> supply with a
screen<strong>in</strong>g test; the heterosexual partners <strong>of</strong> persons with AIDS; <strong>and</strong> children born to<br />
<strong>in</strong>fected mothers.<br />
There is presently no cure for AIDS, although the antiviral agent zidovuz<strong>in</strong>e<br />
(AZT) appears to hold the virus <strong>in</strong> check, at least for a time. Many other antiretroviral<br />
drugs are be<strong>in</strong>g tested, as are agents to bolster the immune system <strong>and</strong> agents to prevent<br />
or treat opportunistic <strong>in</strong>fections. Research on vacc<strong>in</strong>es to prevent the spread <strong>of</strong> AIDS is<br />
also under way.<br />
Cancers <strong>of</strong> the Immune System<br />
Cells <strong>of</strong> the immune system, like those <strong>of</strong> other body systems, can proliferate<br />
uncontrollably; the result is cancer. Leukemias are caused by the proliferation <strong>of</strong> white<br />
<strong>blood</strong> cells, or leukocytes. The uncontrolled growth <strong>of</strong> antibody-produc<strong>in</strong>g (plasma)<br />
cells can lead to multiple myeloma. Cancers <strong>of</strong> the lymphoid organs, known as<br />
lymphomas, <strong>in</strong>clude Hodgk<strong>in</strong>'s disease. These disorders can be treated-some <strong>of</strong> them<br />
very successfully-by drugs <strong>and</strong>/or irradiation.
The Human Immune Response System<br />
An overview <strong>of</strong> the system
An overview <strong>of</strong> the system<br />
The human immune response system recognizes pathogens <strong>and</strong> acts to remove,<br />
immobilize, or neutralize them. The immune system is antigen-specific (respond<strong>in</strong>g to<br />
specific molecules on a pathogen) <strong>and</strong> has memory (its defense to a pathogen is<br />
encoded for future activation). The immune system relies on several <strong>components</strong> to<br />
fight an <strong>in</strong>fect<strong>in</strong>g pathogen. T cells are lymphocytes that circulate between the <strong>blood</strong>,<br />
lymph, <strong>and</strong> lymphoid organs to trigger a systemic immune response with antigen-<br />
receptors on the T cell membrane. B cells are lymphocytes that activate the primary<br />
immune response when antigens b<strong>in</strong>d to their receptors, caus<strong>in</strong>g the B cells to<br />
proliferate. Daughter cells <strong>of</strong> B cells later differentiate <strong>in</strong>to antibody-releas<strong>in</strong>g plasma<br />
cells. B cells also comprise the immune system's memory (see diagram).<br />
Antibodies, also called immunoglobul<strong>in</strong>s, are divided <strong>in</strong>to five classes by structure<br />
<strong>and</strong> function, enabl<strong>in</strong>g them to recognize a wide spectrum <strong>of</strong> antigens. Antibody<br />
functions <strong>in</strong>clude complement fixation that can lead to antigen-cell lysis (rupture) <strong>and</strong><br />
can cause <strong>in</strong>flammation. Antibodies also generate a neutralization response where<br />
viruses <strong>and</strong> bacteria are destroyed by phagocytes. Agglut<strong>in</strong>ation, or clump<strong>in</strong>g together,<br />
<strong>of</strong> foreign cells are caused by B cells' promotion <strong>of</strong> complex cross-l<strong>in</strong>k<strong>in</strong>g <strong>of</strong> antibodies<br />
b<strong>in</strong>d<strong>in</strong>g to antigens. These agglut<strong>in</strong>ated cells are phagocytized. B cells are cloned <strong>in</strong><br />
massive quantities for a s<strong>in</strong>gle specific antigen.<br />
Immune response to T. cruzi<br />
The human immune response to T. cruzi <strong>in</strong>fection is <strong>in</strong>adequate; it provides only a<br />
partial defense at best. The immune system's response at its worst causes the defense
mechanisms to turn on the body it is <strong>in</strong>tended to protect, thus <strong>of</strong>ten caus<strong>in</strong>g more harm<br />
to the person than does T. cruzi.
As T. cruzi immunizes humans to their own antigens, human antibodies attack<br />
myocardial <strong>and</strong> neural cells.<br />
Complement <strong>in</strong> humans does not become activated solely by T. cruzi <strong>in</strong>vasion;<br />
antibodies must be present for complement to b<strong>in</strong>d to a specific T. cruzi antigen. This<br />
allows T. cruzi to have time to <strong>in</strong>fect human tissue. Parasite stra<strong>in</strong> <strong>and</strong> an <strong>in</strong>dividual's<br />
immune competence are prime factors <strong>in</strong> determ<strong>in</strong><strong>in</strong>g the T. cruzi's pathology <strong>of</strong> an<br />
<strong>in</strong>dividual.<br />
Once <strong>in</strong>fected with T. cruzi, humans acquire partial immunity or resistance to the<br />
severe pathologies <strong>of</strong> Chagas' disease's acute phase through subsequent <strong>in</strong>fections <strong>of</strong> T.<br />
cruzi. This guards many <strong>in</strong>dividuals who live <strong>in</strong> highly endemic areas from the acute<br />
symptoms <strong>of</strong> chagas. Complete removable <strong>of</strong> the parasite from these <strong>in</strong>dividuals would<br />
risk the onset <strong>of</strong> acute chagas through future <strong>in</strong>fection, which is deadly - especially for<br />
children.<br />
T. cruzi <strong>in</strong>corporates certa<strong>in</strong> host cell membrane prote<strong>in</strong>s onto its surface thereby<br />
mask<strong>in</strong>g its antigenic signal to the immune system's lymphocytes. T. cruzi can also<br />
cleave antibody molecules on its surface thereby escap<strong>in</strong>g the immune response's<br />
detection. T. cruzi frequently <strong>in</strong>vade monocytes, a circulat<strong>in</strong>g phagocyte. Intracellular<br />
phagocytosis br<strong>in</strong>g amastigotic T. cruzi <strong>in</strong>to tissue cells where they can proliferate.<br />
Once <strong>in</strong>side tissue cells, T. cruzi are undetected by immune response. Trypomastigotes<br />
rema<strong>in</strong> <strong>in</strong> the <strong>blood</strong> stream for a short period <strong>of</strong> time so that the T. cruzi-specific<br />
immunoglobul<strong>in</strong>s don't have sufficient time to be activated. T. cruzi employs successful<br />
strategies to escape the remarkably potent immune response system. By mask<strong>in</strong>g<br />
themselves or by elud<strong>in</strong>g the response mechanisms, the parasite is able to adapt to<br />
survive <strong>and</strong> cont<strong>in</strong>ue the life <strong>of</strong> the species.<br />
Immune response that damages the human body<br />
Un<strong>in</strong>tentional damage is done to the body's otherwise healthy tissue as the<br />
response system attacks what it recognizes as a trigger for a defensive response but
does not recognize that it is attack<strong>in</strong>g itself. This is what's known as an autoimmune<br />
reaction.<br />
Autoimmune responses are responsible <strong>in</strong> large part for the destructive symptoms<br />
<strong>of</strong> Chagas disease. This pathology is referred to as immunopathology. Severe<br />
<strong>in</strong>flammation occurs around tissue that embody amastigotes as the amastigotes release<br />
themselves from the tissue's dead cells. Among the tissue most <strong>of</strong>ten encysted is<br />
myocardial neural plexes. Plexes are networks <strong>of</strong> nerves that serve a variety <strong>of</strong> organs<br />
<strong>and</strong> functions. Digestive system neural plexes are targets as well, namely <strong>in</strong> the colon<br />
<strong>and</strong> esophagus. Dur<strong>in</strong>g the acute phase <strong>of</strong> chagas, B <strong>and</strong> T cells are <strong>in</strong>cited to produce<br />
antibodies. S<strong>in</strong>ce T. cruzi is able to mask its presence <strong>in</strong> the <strong>blood</strong>, these antibodies do<br />
not attack T. cruzi but <strong>in</strong>stead go after cell membrane antigenic <strong>components</strong> called<br />
epitopes, that the body's healthy cells <strong>and</strong> T. cruzi share. Research is be<strong>in</strong>g done to<br />
isolate the epitope <strong>and</strong> how T. cruzi uses it to elude recognition by the immune system.<br />
Scientists work to f<strong>in</strong>d a cure to T. cruzi's <strong>in</strong>fect<strong>in</strong>g the human species. As research<br />
cont<strong>in</strong>ues <strong>in</strong>to how T. cruzi uses the human body as a host, the discipl<strong>in</strong>es <strong>of</strong><br />
parasitology <strong>and</strong> immunology learn much about how these organisms adapt <strong>and</strong> thrive<br />
<strong>in</strong> chang<strong>in</strong>g environments. T. cruzi proves to be a formidable opponent <strong>in</strong> the fight.
Mediated by Macrophages<br />
Mediated by Lymphocytes <strong>and</strong> mast cells
B-Cells<br />
Identical <strong>in</strong> all Individuals Depends on Exposure to Pathogens<br />
Fixed by Evolution<br />
Evolv<strong>in</strong>g with<strong>in</strong> <strong>in</strong>dividuals<br />
Parasites, worms, chemicals, tox<strong>in</strong>s generic viruses, bacteria lipoprote<strong>in</strong>s,<br />
lipocarbohydrates bacterial DNA
Phagocytosis (engulfment)<br />
Activation <strong>of</strong> Antibodies
CD8 Cells