The report is available in English with a Dutch summary - KCE
The report is available in English with a Dutch summary - KCE
The report is available in English with a Dutch summary - KCE
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
30 Plasma <strong>KCE</strong> Reports 120<br />
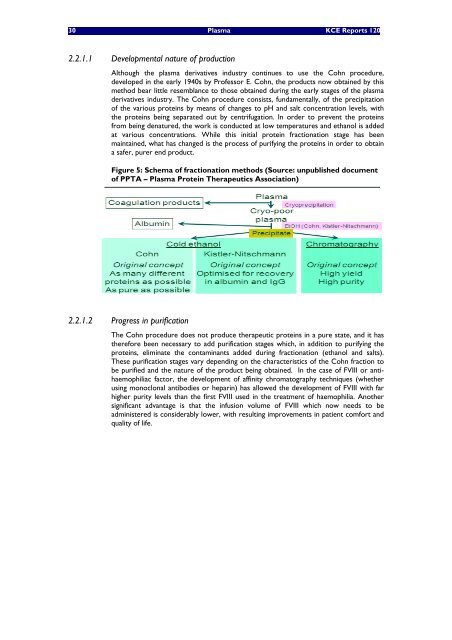
2.2.1.1 Developmental nature of production<br />
Although the plasma derivatives <strong>in</strong>dustry cont<strong>in</strong>ues to use the Cohn procedure,<br />
developed <strong>in</strong> the early 1940s by Professor E. Cohn, the products now obta<strong>in</strong>ed by th<strong>is</strong><br />
method bear little resemblance to those obta<strong>in</strong>ed dur<strong>in</strong>g the early stages of the plasma<br />
derivatives <strong>in</strong>dustry. <strong>The</strong> Cohn procedure cons<strong>is</strong>ts, fundamentally, of the precipitation<br />
of the various prote<strong>in</strong>s by means of changes to pH and salt concentration levels, <strong>with</strong><br />
the prote<strong>in</strong>s be<strong>in</strong>g separated out by centrifugation. In order to prevent the prote<strong>in</strong>s<br />
from be<strong>in</strong>g denatured, the work <strong>is</strong> conducted at low temperatures and ethanol <strong>is</strong> added<br />
at various concentrations. While th<strong>is</strong> <strong>in</strong>itial prote<strong>in</strong> fractionation stage has been<br />
ma<strong>in</strong>ta<strong>in</strong>ed, what has changed <strong>is</strong> the process of purify<strong>in</strong>g the prote<strong>in</strong>s <strong>in</strong> order to obta<strong>in</strong><br />
a safer, purer end product.<br />
Figure 5: Schema of fractionation methods (Source: unpubl<strong>is</strong>hed document<br />
of PPTA – Plasma Prote<strong>in</strong> <strong>The</strong>rapeutics Association)<br />
2.2.1.2 Progress <strong>in</strong> purification<br />
<strong>The</strong> Cohn procedure does not produce therapeutic prote<strong>in</strong>s <strong>in</strong> a pure state, and it has<br />
therefore been necessary to add purification stages which, <strong>in</strong> addition to purify<strong>in</strong>g the<br />
prote<strong>in</strong>s, elim<strong>in</strong>ate the contam<strong>in</strong>ants added dur<strong>in</strong>g fractionation (ethanol and salts).<br />
<strong>The</strong>se purification stages vary depend<strong>in</strong>g on the character<strong>is</strong>tics of the Cohn fraction to<br />
be purified and the nature of the product be<strong>in</strong>g obta<strong>in</strong>ed. In the case of FVIII or antihaemophiliac<br />
factor, the development of aff<strong>in</strong>ity chromatography techniques (whether<br />
us<strong>in</strong>g monoclonal antibodies or hepar<strong>in</strong>) has allowed the development of FVIII <strong>with</strong> far<br />
higher purity levels than the first FVIII used <strong>in</strong> the treatment of haemophilia. Another<br />
significant advantage <strong>is</strong> that the <strong>in</strong>fusion volume of FVIII which now needs to be<br />
adm<strong>in</strong><strong>is</strong>tered <strong>is</strong> considerably lower, <strong>with</strong> result<strong>in</strong>g improvements <strong>in</strong> patient comfort and<br />
quality of life.