Growth of Intermetallic Compounds in Thermosonic Copper Wire ...
Growth of Intermetallic Compounds in Thermosonic Copper Wire ...
Growth of Intermetallic Compounds in Thermosonic Copper Wire ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Growth</strong> <strong>of</strong> <strong>Intermetallic</strong> <strong>Compounds</strong> <strong>in</strong> <strong>Thermosonic</strong> <strong>Copper</strong> <strong>Wire</strong> Bond<strong>in</strong>g on Alum<strong>in</strong>um Metallization 129<br />
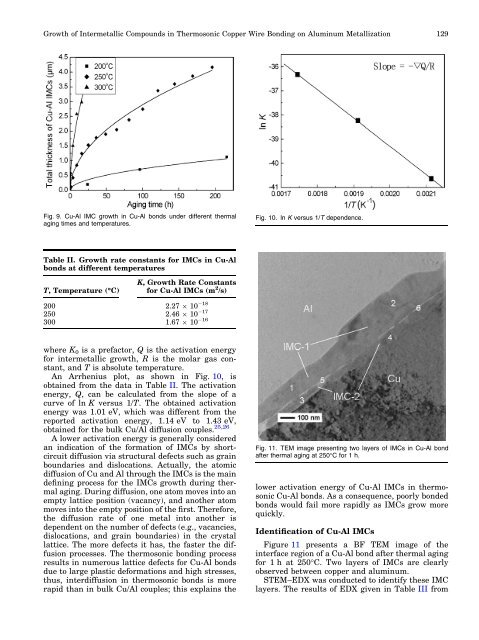
Fig. 9. Cu-Al IMC growth <strong>in</strong> Cu-Al bonds under different thermal<br />
ag<strong>in</strong>g times and temperatures.<br />
Table II. <strong>Growth</strong> rate constants for IMCs <strong>in</strong> Cu-Al<br />
bonds at different temperatures<br />
T, Temperature (°C)<br />
K, <strong>Growth</strong> Rate Constants<br />
for Cu-Al IMCs (m 2 /s)<br />
200 2.27 9 10 18<br />
250 2.46 9 10 17<br />
300 1.67 9 10 16<br />
where K 0 is a prefactor, Q is the activation energy<br />
for <strong>in</strong>termetallic growth, R is the molar gas constant,<br />
and T is absolute temperature.<br />
An Arrhenius plot, as shown <strong>in</strong> Fig. 10, is<br />
obta<strong>in</strong>ed from the data <strong>in</strong> Table II. The activation<br />
energy, Q, can be calculated from the slope <strong>of</strong> a<br />
curve <strong>of</strong> ln K versus 1/T. The obta<strong>in</strong>ed activation<br />
energy was 1.01 eV, which was different from the<br />
reported activation energy, 1.14 eV to 1.43 eV,<br />
obta<strong>in</strong>ed for the bulk Cu/Al diffusion couples. 25,26<br />
A lower activation energy is generally considered<br />
an <strong>in</strong>dication <strong>of</strong> the formation <strong>of</strong> IMCs by shortcircuit<br />
diffusion via structural defects such as gra<strong>in</strong><br />
boundaries and dislocations. Actually, the atomic<br />
diffusion <strong>of</strong> Cu and Al through the IMCs is the ma<strong>in</strong><br />
def<strong>in</strong><strong>in</strong>g process for the IMCs growth dur<strong>in</strong>g thermal<br />
ag<strong>in</strong>g. Dur<strong>in</strong>g diffusion, one atom moves <strong>in</strong>to an<br />
empty lattice position (vacancy), and another atom<br />
moves <strong>in</strong>to the empty position <strong>of</strong> the first. Therefore,<br />
the diffusion rate <strong>of</strong> one metal <strong>in</strong>to another is<br />
dependent on the number <strong>of</strong> defects (e.g., vacancies,<br />
dislocations, and gra<strong>in</strong> boundaries) <strong>in</strong> the crystal<br />
lattice. The more defects it has, the faster the diffusion<br />
processes. The thermosonic bond<strong>in</strong>g process<br />
results <strong>in</strong> numerous lattice defects for Cu-Al bonds<br />
due to large plastic deformations and high stresses,<br />
thus, <strong>in</strong>terdiffusion <strong>in</strong> thermosonic bonds is more<br />
rapid than <strong>in</strong> bulk Cu/Al couples; this expla<strong>in</strong>s the<br />
Fig. 10. ln K versus 1/T dependence.<br />
Fig. 11. TEM image present<strong>in</strong>g two layers <strong>of</strong> IMCs <strong>in</strong> Cu-Al bond<br />
after thermal ag<strong>in</strong>g at 250°C for 1 h.<br />
lower activation energy <strong>of</strong> Cu-Al IMCs <strong>in</strong> thermosonic<br />
Cu-Al bonds. As a consequence, poorly bonded<br />
bonds would fail more rapidly as IMCs grow more<br />
quickly.<br />
Identification <strong>of</strong> Cu-Al IMCs<br />
Figure 11 presents a BF TEM image <strong>of</strong> the<br />
<strong>in</strong>terface region <strong>of</strong> a Cu-Al bond after thermal ag<strong>in</strong>g<br />
for 1 h at 250°C. Two layers <strong>of</strong> IMCs are clearly<br />
observed between copper and alum<strong>in</strong>um.<br />
STEM–EDX was conducted to identify these IMC<br />
layers. The results <strong>of</strong> EDX given <strong>in</strong> Table III from