Chapter 38 Workbook Solutions
Chapter 38 Workbook Solutions
Chapter 38 Workbook Solutions
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>38</strong>-1 0 cHAPTER <strong>38</strong> . Quantization<br />
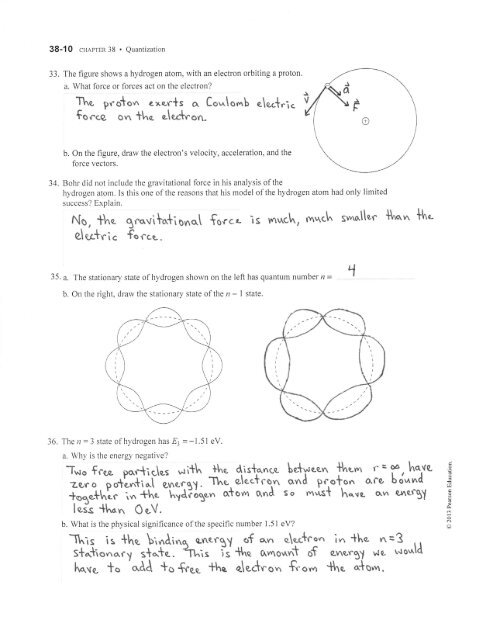
33. The figure shows a hydrogen atom, with an electron orbiting a proton.<br />
a. What force or forces act on the electron?<br />
Thc pro*o,^ exor+s a Co.^lo*b e\ec+r'r<<br />
focce on tlne. olert'on-<br />
b. On the figure, draw the electron's velocity, acceleration, and the<br />
force vectors.<br />
34. Bohr did not inclr-rde the gravitational force in his analysis of the<br />
hydrogen atom. Is this one of the reasons that his modelof the hydrogen atom had only limited<br />
success? Explain.<br />
No, thu grovilati onq\ fun..<br />
e\er*r i c fo rce .<br />
35. a. The stationary state of hydrogen shown on the left has quantum number n =<br />
b. On the right, draw the stationary state of the r -<br />
36. The n = 3 state of hydrogen has E3 = -l .51 eV.<br />
a. Why is the energy negative?<br />
1 state.<br />
Jwo Fcw pqc*is\e,s $a. dis*o.ncc<br />
;;; "ri+h<br />
Zgro. " i'"+L^ii^i' po*cnti .*"q$Y- q$y. Th.., electcon<br />
'I1nc, ele.tcon<br />
iu,["'i; +h; -irril*. atom qnA<br />
*oqethcc in tha<br />
leJs *hon O e-V.<br />
b. What is the physical significance of the specific number 1.51 eV?<br />
\,s i s *he \ir,J,irl,o q-nef qy of o.n c\ec+co''<br />
s{a*iono,ry s***e. 'ruit ir +ho hrrrovrnt of<br />
ho.vQ, *o 'oAd *o &ee *ha. e\ec*ro". to*<br />
ls h^\,\ch, rn\ch s^nltor {Lo.'n tho.<br />
q<br />
tetw"on *h"t"t r'= oa hqvq,<br />
or,d Dcoton afo<br />
S o ^"St horr. o.rn<br />
b J.^"d<br />
Ql^efsY<br />
i'' *ho n =3<br />
a\e,r3Y $e *oold<br />
*ho o4orn.<br />
d<br />
I<br />
=<br />
E<br />
o<br />
o<br />
o.<br />
d<br />
o