Bioseed - Biotissue Technologies Gmbh
Bioseed - Biotissue Technologies Gmbh
Bioseed - Biotissue Technologies Gmbh
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Table of contents<br />
Introduction 5<br />
Characteristics 6<br />
Clinical effectiveness 9<br />
Safety 15<br />
Indications 17<br />
Surgical technique 18<br />
Clinical cases 24<br />
Forms 28<br />
Bibliography 30<br />
3<br />
BioSeed ® -C<br />
For Articular Cartilage<br />
Regeneration
Three-dimensional polymer carrier<br />
Fleece with CE marking<br />
Approx. 20x10 6 cells per cm 3<br />
Innovative, exclusive, secure fixation<br />
Optimum primary stability of the implant<br />
Cultivation with autologous serum in GMP laboratory<br />
Can be implanted up to 21 days after removal<br />
Any transplantation dates possible thanks to cryoconservation<br />
Can be delivered all over Europe<br />
More than 300 patients successfully treated<br />
Chondrocytes<br />
(Illustration)<br />
5<br />
BioSeed ® -C<br />
Tissue Regeneration Technique<br />
Thanks to autologous chondrocyte transplantation<br />
with 3D matrix, knee injuries (femoral<br />
condyle, tibia plateau, patella), ankle injuries<br />
(talus) or hip injuries (acetabulum) can be<br />
treated with the patient’s own cells. For this<br />
procedure, cells are multiplied in the laboratory,<br />
and then re-transplanted into the damaged<br />
area.<br />
Hyaline articular cartilage cannot regenerate<br />
itself. However, chondrocytes (cartilage cells)<br />
can be multiplied in the laboratory with<br />
corresponding stimulation; they are re-used<br />
in the body and develop into hyaline articular<br />
cartilage.<br />
Before chondrocyte transplantation, a small<br />
amount of cartilage tissue is arthroscopically<br />
removed from a healthy area that is under<br />
less strain from the knee. The cells are then<br />
cultivated for three weeks in vitro and in this<br />
time they are absorbed into a three-dimensional<br />
matrix. The matrix used consists of two<br />
components. These are a carrier fleece which<br />
forms the framework structure and a biological<br />
“glue” which fixes the homogenously<br />
distributed cells in the carrier fleece. This<br />
combination of the two matrix components<br />
provides the chondrocytes with an ideal threedimensional<br />
environment which stimulates<br />
them to redifferentiate and thus assume their<br />
original morphology and function. As a result,<br />
the graft itself is mechanically stable,<br />
supple, flexible and can be cut to fit the size<br />
of defect. Due to these special properties,<br />
a new fixation technique (transosseous fixation)<br />
and arthroscopic transplantation are<br />
possible, and the indication can be extended.<br />
BioSeed ® -C is intended for the treatment of<br />
extensive cartilage defects.<br />
Chondrocyte transplantation is mainly carried<br />
out on patients under 60 who suffer from<br />
constant pain and impaired mobility because<br />
of individual or multiple cartilage damage,<br />
measuring over 1.5 cm 2 , caused by traumas or<br />
focal degenerative changes.
Photo 01<br />
Photo 02<br />
BioSeed ® -C<br />
Characteristics<br />
Autologous chondrocyte transplantation is<br />
nothing new. In studies published by Brittberg<br />
et al. (17) in 1994, clinical application of<br />
autologous chondrocytes for treating cartilage<br />
defects was proven in over 5000 cases. The<br />
results were presented in various publications<br />
(15, 16). The findings of medium- and longterm<br />
studies are particularly promising for<br />
patients who have undergone unsuccessful<br />
cartilage treatment in the past.<br />
A feature of the original technique was the<br />
injection of chondrocyte suspension into the<br />
interior of a “bioactive chamber” under a<br />
periosteal “flap”. Now, technical innovations in<br />
cell culture mean that chondrocytes can be<br />
expanded in a three-dimensional resorbable<br />
carrier fleece, which has resulted in standardised<br />
cell distribution and a simplified implantation<br />
technique (1, 2, 3, 4, 5). The quality of<br />
newly formed hyaline cartilage was researched<br />
in animal experiments (6, 8, 9, 10, 11, 12, 13).<br />
Preclinical tests in horses, for example, show<br />
the characteristic structure of hyaline articular<br />
cartilage 24 months after transplantation of<br />
BioSeed ® -C (7/Photo 04).<br />
6<br />
Photo 03<br />
Photo 04<br />
7<br />
A unit of BioSeed ® -C consists of two rectangular<br />
three-dimensional carrier fleeces,<br />
consisting of resorbable suture materials and<br />
measuring 30 mm long, 20 mm wide and<br />
2 mm high. The carrier fleece contains interconnecting<br />
cavities (over 90 % of the total<br />
volume) where the cultivated cells are evenly<br />
distributed using fibrin glue. The cell density<br />
is 20 x 10 6 /cm 3 .<br />
Due to the properties of the carrier fleece,<br />
BioSeed ® -C has excellent primary mechanical<br />
stability as well as perfect biocompatibility.<br />
In addition, thanks to this matrix, the cells are<br />
distributed homogenously in a three-dimensional<br />
manner, with the chondrocyte phenotype<br />
remaining differentiated.<br />
Autologous chondrocytes are obtained by<br />
arthroscopic removal of cartilage then cultivated<br />
for about 21 days according to GMP<br />
(Good Manufacturing Practice) guidelines.<br />
Only autologous blood serum is used during<br />
the process.<br />
The graft can be cut to any shape required<br />
for transplantation using anatomical or surgical<br />
scissors.<br />
BioSeed ® -C is the result of years of research<br />
carried out by Berlin Charité University of<br />
Medicine and by Freiburg University Hospital.
9<br />
BioSeed ® -C<br />
Clinical effectiveness<br />
Results 6, 12 and 24 months after transplantation<br />
of BioSeed ® -C to treat cartilage<br />
defects of the knee.<br />
This prospective study carried out by Dr. C.<br />
Erggelet from the Department of Orthopaedic<br />
Surgery, Freiburg University Hospital, Germany<br />
began in November 2001. Clinical evaluation<br />
and MRIs were carried out at the following<br />
intervals:<br />
• when BioSeed ® -C was transplanted<br />
• at the 3 month follow-up<br />
• at the 6 month follow-up<br />
• at the 12 month follow-up<br />
• at the 24 month follow-up
Photo 05<br />
Example of<br />
a MRI scan<br />
6 months<br />
after transplantation<br />
of<br />
BioSeed ® -C<br />
into the<br />
femoral<br />
condyle<br />
Photo 06<br />
Second look<br />
arthroscopy<br />
9 months<br />
after transplantation<br />
Transplant<br />
Method<br />
By November 2004, a total of 79 patients<br />
(30 women, 49 men) had been treated with<br />
BioSeed ® -C. The average age of patients was<br />
36 (age range 17 - 64). 42 patients had cartilage<br />
defects in the left knee, 37 patients in<br />
the right knee. All defects were grade IV in<br />
Outerbridge's classification (= cartilage defect<br />
down to the bone). Most of the defects were<br />
situated on the medial femoral condyle. In<br />
17 cases BioSeed ® -C was implanted in the<br />
retropatellar region, in six cases in the trochlea<br />
and in three cases on the lateral femoral<br />
condyle. In one case the transplantation site<br />
was the medial tibia plateau. 21 patients<br />
suffered from two defects: five lesions were<br />
situated on the medial femoral condyle, three<br />
on the lateral femoral condyle, eight on the<br />
trochlea, and five on the patella.<br />
Concomitant surgery was performed in<br />
34 cases, as the following table shows:<br />
Concomitant surgery Number<br />
Micro-fractures 5<br />
ACL reconstruction 4<br />
Repositioning osteotomy 21<br />
Medial capsular shift<br />
Lateral release (transection<br />
2<br />
of the lateral retinaculum) 2<br />
In 71 cases, the transplantation of BioSeed ® -C<br />
was performed by arthrotomy, and in eight<br />
cases by arthroscopy. The results were assessed<br />
according to the IKDC (International Knee<br />
Documentation Committee), KOOS (Knee<br />
Osteoarthritis Outcome Score) and modified<br />
Cincinnati Knee Score.<br />
10<br />
Photo 07<br />
Histology 9<br />
months after<br />
transplantation<br />
Photo 08<br />
Histology<br />
magnified<br />
100 times<br />
11<br />
Results<br />
The MRI scans of all patients at 6 months<br />
after surgery show good integration of the<br />
graft and satisfactory cover of the defect.<br />
The graft has bonded extremely well to the<br />
surrounding articular cartilage and to the<br />
sub-chondral bone. There is still a visible contrast<br />
in colour to the surrounding cartilage,<br />
and the transosseous drill holes can be seen.<br />
There are no signs of articular constriction<br />
or loosening or separating of the graft<br />
(Photo 05).<br />
Histology<br />
This biopsy taken 9 months after implantation<br />
of BioSeed ® -C showed that, histologically,<br />
the graft had integrated well with a firm bond<br />
to the surrounding bone. The characteristic<br />
structure of the hyaline cartilage can be observed<br />
(Photo 07, 08).
Diagram A<br />
Diagram B<br />
Clinical findings<br />
By November 2004 3-month results were<br />
available for 60 patients (3 mo), 6-month<br />
results for 66 patients (6 mo), 12-month results<br />
for 60 patients (12 mo) and 24-month<br />
results for 40 patients (24 mo).<br />
Modified Cincinnati Knee Score<br />
Diagram A shows mean value (mean) and<br />
standard deviation (std) of the score assessed<br />
by doctor and patient. A score of 10<br />
represents the best state of health.<br />
IKDC Score (joint effusion)<br />
Diagram B shows the percentage of patients<br />
with knee joint effusion.<br />
12<br />
Diagram C<br />
Diagram D<br />
13<br />
IKDC Score (subjective knee<br />
evaluation form)<br />
The data in Diagram C are obtained by adding<br />
together the scores for various items. The<br />
score ranges from 0 to 100. The results are<br />
interpreted as a function measurement of the<br />
knee. 100 points on the scale represent unlimited<br />
activity in day-to-day life and a complete<br />
lack of symptoms.<br />
KOOS Score<br />
Diagram D shows changes in each item of<br />
the KOOS evaluation 24 months after surgery.<br />
The score ranges from 0 to 100. 100 is the<br />
best possible state of health. All evaluated<br />
patients are included in the analysis.
Official approval to manufacture drugs using material of human origin.<br />
Photo 09<br />
Production<br />
in the GMPclean<br />
room<br />
laboratories<br />
Photo 10<br />
15<br />
BioSeed ® -C<br />
Safety<br />
BioSeed ® -C is produced from the cartilage<br />
biopsy and blood serum taken from the patient.<br />
At the start of cultivation, the patient’s<br />
blood is analysed in the laboratory for virus<br />
serology.<br />
The carrier fleece needed to cultivate<br />
BioSeed ® -C is completely biocompatible and<br />
consists of surgical, resorbable suture<br />
material.<br />
The fibrin glue, an authorised drug, used in<br />
BioSeed ® -C, is optimised in the laboratory to<br />
match the requirements of the cells. GMP<br />
guidelines (Good Manufacturing Practice) are<br />
followed in the cultivation of BioSeed ® -C.<br />
The 3D chondrocyte grafts are produced<br />
according to European legislation in a GMP<br />
laboratory, specially developed for cell<br />
cultivation and managed under strict criteria.<br />
A licence to manufacture human drugs is<br />
necessary to produce grafts.
Classification according to Outerbridge<br />
Photo 11<br />
17<br />
I II III IV<br />
BioSeed ® -C<br />
Indications<br />
The indications for BioSeed ® -C are: Chondral<br />
traumatic and focally degenerative defects<br />
(grade III and IV in Outerbridge's classification),<br />
measuring over 1.5 cm 2 .<br />
Treatment with BioSeed ® -C is recommended<br />
for application in the femoral condyle, patella,<br />
trochlea, tibia plateau, talus and acetabulum.<br />
BioSeed ® -C can be transplanted while performing<br />
other types of surgery, such as microfractures,<br />
crucial ligament reconstruction,<br />
osteotomy, medial capsular shift and lateral<br />
release.<br />
BioSeed ® -C is indicated for patients under<br />
60 years of age, although there is no age<br />
limit for cultivation.<br />
There should be no previous or acute infections<br />
of the joint.<br />
The patient should not be suffering from an<br />
acute infectious disease, metabolic systemic<br />
diseases or diseases of the immune system.<br />
The same applies to neoplasia.
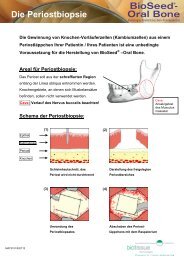
Phase 01<br />
Phase 02<br />
BioSeed ® -C<br />
Surgical technique<br />
The application of BioSeed ® -C takes place<br />
in two stages: biopsy of cartilage and<br />
transplantation of BioSeed ® -C.<br />
First stage: Arthroscopic biopsy of cartilage<br />
The cartilage biopsy is removed using<br />
arthroscopy. Approx. 250 mg of cartilage<br />
tissue (the equivalent of about six grains of<br />
rice) is removed from a healthy area subject<br />
to less stress (Phase 01).<br />
The cartilage biopsy is placed in a special<br />
biopsy tube (which is then packed in a sterile<br />
larger tube) that contains the liquid for<br />
transportation (Phase 02).<br />
18<br />
Phase 03<br />
Phase 04<br />
19<br />
Approx. 100 ml of blood is collected in order<br />
to cultivate the chondrocytes in the laboratory<br />
(Phase 03).<br />
The biopsy tube and monovettes with the<br />
patient’s chondrocytes and blood are placed<br />
in an insulated container for transportation<br />
(Phase 04).<br />
Together with the completed forms, the patient's<br />
own biopsy and blood are sent to the<br />
laboratory within 72 hours of removal to<br />
begin the cultivation of chondrocytes.
Phase 01<br />
Phase 02<br />
Second stage: transplantation of<br />
BioSeed ® -C<br />
Preparation<br />
The defective zone is prepared using a curette.<br />
In this procedure, the area is cleaned down<br />
to the sub-chondral bone using debridement.<br />
Depending on the defect, a geometrical (rectangular,<br />
triangular or trapezoidal) form is<br />
prepared, which ensures that the graft has<br />
optimum primary stability (Phase 01). It is<br />
advisable to remove all the damaged cartilage<br />
tissue. If the area to be treated is very deep,<br />
two grafts can be placed on top of each other.<br />
For even deeper defects, the sub-chondral<br />
bone can be built up with spongiosa, and the<br />
chondrocyte graft applied.<br />
The prepared area is measured and<br />
BioSeed ® -C is cut to the right size (Phase<br />
02/03).<br />
Implantation<br />
BioSeed ® -C can be fixed in different ways:<br />
For treating large defects or defects without<br />
marginal cartilage, a graft should be fixed<br />
in a stable and secure manner. The anchoring<br />
technique using knots is recommended for<br />
this procedure.<br />
20<br />
Phase 03<br />
Phase 04<br />
21<br />
Anchoring technique using knots (transosseous<br />
fixation or Erggelet technique)<br />
This technique comprises transosseous drill<br />
holes and anchorage by knots. The transosseous<br />
drill holes are produced inserting four<br />
Kirschner wires (Ø 1.7 mm) through the four<br />
corners of the defect site (Phase 04).
Phase 05<br />
Phase 06<br />
Phase 07<br />
Knot technique<br />
Using resorbable surgical thread (Vicryl 2 - 0),<br />
first pull the thread diagonally through one<br />
corner of BioSeed ® -C. The first knot, the anchoring<br />
knot, is positioned about 0.5 – 1 cm<br />
from the graft and the Vicryl thread is<br />
blocked with a clamp. Then one knot, made<br />
of three knots arranged on top of each other,<br />
is formed. Each of these knots consists of<br />
four alternating loops. This three-fold knot<br />
(Ø 1.7 mm) secures fixation in the drilling<br />
channel.<br />
With another clamp, a distance of 0.5 – 1 cm<br />
is set again and the Vicryl thread blocked.<br />
Then a knot, consisting of two knots each<br />
with three alternating loops, is set (Phase 05).<br />
The second knot forms a pulley loop, through<br />
which a pulley thread (Vicryl thread) is later<br />
fed, which in turn is used for pulling in the<br />
anchoring knot (Phase 06). The same procedure<br />
is repeated at all four corners of the graft.<br />
22<br />
Phase 08<br />
Phase 09<br />
Photo 12<br />
23<br />
The pulley threads are pulled through the<br />
prepared loop, then both ends of the pulley<br />
threads are pulled through the loops of the<br />
four Kirschner wires. The graft is then pulled<br />
in through the condyles (Phase 07).<br />
The graft is securely anchored in the defect<br />
area (Phase 08). The pulley threads are pulled<br />
out at the subcutaneous exit.<br />
The same technique can be carried out by<br />
arthroscopy (Phase 09).<br />
Instruments specially designed for this purpose<br />
are available for arthroscopic transplantation.<br />
Sewing in the graft (Fichtl's method)<br />
BioSeed ® -C can also be sewn into the defect.<br />
It is sewn in securely by fixation using the<br />
single knot method at the corners and two<br />
other places on the graft. The suture material<br />
used for this procedure is PDS 6-0.<br />
Fixation with fibrin glue<br />
For this method, after preparation, all that<br />
is needed is to apply fibrin glue to the side<br />
of the defect, position the graft and press<br />
down lightly with the fingers for about three<br />
minutes.
Photo 13<br />
Photo 15<br />
Photo 17<br />
BioSeed ® -C<br />
Clinical cases<br />
Photo 14<br />
Photo 16<br />
Photo 18<br />
Femoral condyle<br />
41-year-old patient with post-traumatic<br />
lesion of the medial femoral condyle<br />
Photo 13 Cartilage defect<br />
Photo 14 BioSeed ® -C implant armed<br />
with 4 vicryl 2-0- threads<br />
Photo 15 Transosseous fixation of<br />
the graft<br />
Photo 16 Transplanted BioSeed ® -C<br />
By kind permission of Dr. P. Bachelin,<br />
Genolier<br />
34-year-old patient with traumatic<br />
lesion of the medial femoral condyle<br />
Photo 17 Measuring the size of<br />
the defect by taking an impression<br />
using film<br />
Photo 18 Transplanted BioSeed ® -C<br />
By kind permission of Dr. F. Zeifang,<br />
Heidelberg<br />
24<br />
25<br />
Photo 19<br />
Photo 21<br />
Photo 23<br />
Photo 20<br />
Photo 22<br />
Photo 24<br />
Arthroscopic transplantation in<br />
femoral condyle<br />
24-year-old patient with traumatic<br />
cartilage defect<br />
Photo 19 Arthroscopic view of the<br />
debrided defect<br />
Photo 20 Pulling in the graft<br />
Photo 21 Anchoring the knots<br />
Photo 22 Transplanted BioSeed ® -C<br />
Arthroscopic transplantation in tibia<br />
41-year-old patient with degenerative<br />
cartilage defect<br />
Photo 23 Debrided defect<br />
Photo 24 BioSeed ® -C transplanted<br />
arthroscopically<br />
By kind permission of Dr. C. Erggelet,<br />
Freiburg
Photo 25 Photo 26<br />
Photo 27<br />
Photo 29<br />
Photo 28<br />
Photo 30<br />
Arthroscopic transplantation in<br />
acetabulum<br />
42-year-old patient with traumatic<br />
defect of acetabulum<br />
Photo 25 Defective area in acetabulum<br />
Photo 26 Graft fixed by adhesion<br />
By kind permission of Dr. A. Fontana,<br />
Monza<br />
Patella<br />
26-year-old patient with traumatic defect<br />
due to partial dislocation of the patella<br />
Photo 27 Retropatellar lesion<br />
Photo 28 Measuring the defect<br />
Photo 29 Cutting the graft to size<br />
Photo 30 Transplanted BioSeed ® -C<br />
By kind permission of Prof. J.-L. Rhenter,<br />
Genolier<br />
26<br />
27<br />
Photo 31<br />
Photo 33<br />
Photo 35<br />
Photo 32<br />
Photo 34<br />
Photo 36<br />
Trochlea<br />
41-year-old patient with traumatic<br />
defect of the trochlea<br />
Photo 31 Area of defective cartilage<br />
Photo 32 Transplanted BioSeed ® -C<br />
By kind permission of Dr. F. Zeifang,<br />
Heidelberg<br />
43-year-old patient with advanced<br />
osteoarthritis of the knee<br />
Photo 33 Defect in the trochlea before<br />
transplantation<br />
Photo 34 24 months after transplantation<br />
By kind permission of Dr. J. Holz,<br />
Hamburg<br />
Talus<br />
29-year-old patient with traumatic lesion<br />
of the ankle joint<br />
Photo 35 Cartilage defect<br />
Photo 36 Sewing BioSeed ® -C into<br />
the defect<br />
By kind permission of Dr. W. Fichtl,<br />
Ulm
BioSeed ® -C<br />
Forms<br />
These forms ensure that each biopsy<br />
and blood sample are handled with<br />
the utmost safety. The form needs to<br />
be completed before treatment with<br />
BioSeed ® -C. When doing so, please<br />
comply with the guidelines enclosed<br />
with the biopsy removal kit.<br />
Production order form<br />
Gives details of the patient, the surgeon<br />
and the amount of graft required. Use<br />
the labels enclosed to identify patients.<br />
Form for cryoconservation /<br />
thawing order<br />
Used for requests to conserve tissue<br />
material for a subsequent operation,<br />
sent by fax.<br />
28 29<br />
Patient consent form<br />
Used to obtain the patient's consent for<br />
the blood analysis required for cultivation<br />
and for consent to use personal data.
BioSeed ® -C<br />
Bibliography<br />
1) Erggelet C., Die Behandlung von Gelenkknorpeldefekten,<br />
Steinkopff Verlag Darmstadt;<br />
2004<br />
2) Erggelet C., et al. Implantation of autologous<br />
chondrocytes in a 3D resorbable<br />
polymer fleece for the treatment of cartilage<br />
defects in the knee joint, ICRS Gent; 2004<br />
3) Burmester G., Tissue Engineering gegen<br />
Arthrose; DZKF 9/10; 2003<br />
4) Preis S., et al. Therapy of cartilage defects<br />
by autologous chondrocyte implantation. DZ<br />
Sportmed. 54: 225-228; 2003<br />
5) Erggelet C., et al. The arthroscopic implantation<br />
of autologous chondrocytes for the<br />
treatment of full thickness cartilage defects<br />
of the knee joint, Arthroscopy Vol 19, No 1<br />
W.B; No. 1 (January) 2003. pp 108-110<br />
6) Sittinger M., et al. Current strategies for<br />
cell delivery in cartilage and bone regeneration;<br />
Current Opinion in Biotechnology 2004,<br />
115: 411-418<br />
7) Kaps C., et al. Molekulare Charakterisierung<br />
von gezüchteten humanen dreidimensionalen<br />
Chondrozytentransplantaten; Der Orthopäde<br />
Springer Verlag 2003, DOI 10.1007/s00132-<br />
32-003-0505-3<br />
8) Barnewitz D., et. al. Tissue Engineering:<br />
Neue Behandlungsansätze bei Knorpelveränderungen<br />
bei degenerativen Gelenkerkrankungen<br />
des Pferdes – erste Ergebnisse einer<br />
Langzeitstudie, Berliner Münchner Tierärztliche<br />
Wochenschrift 116, 157-161 (2003),<br />
Blackwell Verlag, Berlin<br />
9) Risbud M., et al. Tissue Engineering:<br />
advances in vitro cartilage generation; Trends<br />
in Biotechnology Vol.20, No. 8 August 2002<br />
10) Perka C., et. al. Chondrozytentransplantationen<br />
in PGLA/Polydioxanon-Vliesen Orthopäde<br />
29, 112-119 (2000), Springer Verlag<br />
11) Perka C., et al. Tissue engineered cartilage<br />
repair using cryopreserved and noncryopreseved<br />
chondrocytes; Clinical Orthopeadics &<br />
related research 2000; 378: 245-254<br />
12) Duda G., et al. Mechanical quality of<br />
tissue engineered cartilage: results after 6<br />
and 12 weeks in vivo. Biomed Mater Res.<br />
53: 673-677 (2000) John Willey & Sons<br />
13) Sittinger M., et al. Resorbable polyesters<br />
in cartilage engineering: affinity and biocompatibility<br />
of polymer fiber structures to chondrocytes.<br />
J Biomed Mater Res 33: 57-63, 1996.<br />
14) Stellungnahme der Arbeitsgemeinschaft<br />
Autologe Chondrozyten Transplantation (ACT)<br />
und Tissue Engineering – unter der Schirmherrschaft<br />
der DGU und der DGOOC; 2002<br />
15) Peterson L., et al. Autologous chondrocyte<br />
transplantation – biomechanics and long<br />
term durability; American J. Sports; Vol. 30<br />
No.1; 2002<br />
16) Peterson L., et al. Two to 9-year outcome<br />
after autologous chondrocyte transplantation<br />
of the knee. Clin Orthop 212-234, 2000<br />
17) Brittberg M., et al. Treatment of deep<br />
cartilage defects in the knee with autologous<br />
chondrocyte transplantation. New England J.<br />
Med. 331:889-895, 1994<br />
Please see the enclosed CD for the bibliography<br />
and the surgical method.<br />
30<br />
BioSeed ® -C<br />
CD
ioSeed®-C<br />
BioTissue <strong>Technologies</strong> GmbH<br />
Engesserstr. 4b<br />
D -79108 Freiburg<br />
Tel. +49(0)761 7676-555<br />
Fax +49 (0) 7 61 76 76 - 430<br />
customerservice@biotissue.de<br />
www.biotissue.de<br />
Autologous three-dimensional<br />
chondrocyte graft