- Page 1: CAHPS ® Hospital Survey (HCAHPS) Q
- Page 4 and 5: ii Centers for Medicare & Medicaid
- Page 6 and 7: HCAHPS Quality Assurance Guidelines
- Page 8 and 9: HCAHPS Quality Assurance Guidelines
- Page 10 and 11: Reader’s Guide March 2011 or cont
- Page 12 and 13: 4 Centers for Medicare & Medicaid S
- Page 14 and 15: Introduction and Overview March 201
- Page 16 and 17: Introduction and Overview March 201
- Page 18 and 19: Introduction and Overview March 201
- Page 22 and 23: Introduction and Overview March 201
- Page 24 and 25: Introduction and Overview March 201
- Page 26 and 27: Program Requirements March 2011 ‣
- Page 28 and 29: Program Requirements March 2011 ‣
- Page 30 and 31: Program Requirements March 2011 Mul
- Page 32 and 33: Program Requirements March 2011 2.
- Page 34 and 35: Program Requirements March 2011 Hos
- Page 36 and 37: Program Requirements March 2011 Tel
- Page 38 and 39: 30 Centers for Medicare & Medicaid
- Page 40 and 41: 32 Centers for Medicare & Medicaid
- Page 42 and 43: Survey Management March 2011 Custom
- Page 44 and 45: Survey Management March 2011 Traini
- Page 46 and 47: Survey Management March 2011 ‣ Im
- Page 48 and 49: Sampling Protocol March 2011 Flowch
- Page 50 and 51: Sampling Protocol March 2011 ‣
- Page 52 and 53: Sampling Protocol March 2011 ‣ If
- Page 54 and 55: Sampling Protocol March 2011 N1 = N
- Page 56 and 57: Sampling Protocol March 2011 Consis
- Page 58 and 59: Sampling Protocol March 2011 o Rand
- Page 60 and 61: Sampling Protocol March 2011 Stratu
- Page 62 and 63: Sampling Protocol March 2011 DSRS E
- Page 64 and 65: Sampling Protocol March 2011 Step D
- Page 66 and 67: Sampling Protocol March 2011 Table
- Page 68 and 69: Mail Only Survey Administration Mar
- Page 70 and 71:
Mail Only Survey Administration Mar
- Page 72 and 73:
Mail Only Survey Administration Mar
- Page 74 and 75:
Mail Only Survey Administration Mar
- Page 76 and 77:
Mail Only Survey Administration Mar
- Page 78 and 79:
70 Centers for Medicare & Medicaid
- Page 80 and 81:
Telephone Only Survey Administratio
- Page 82 and 83:
Telephone Only Survey Administratio
- Page 84 and 85:
Telephone Only Survey Administratio
- Page 86 and 87:
Telephone Only Survey Administratio
- Page 88 and 89:
Telephone Only Survey Administratio
- Page 90 and 91:
Mixed Mode Survey Administration Ma
- Page 92 and 93:
Mixed Mode Survey Administration Ma
- Page 94 and 95:
Mixed Mode Survey Administration Ma
- Page 96 and 97:
Mixed Mode Survey Administration Ma
- Page 98 and 99:
Mixed Mode Survey Administration Ma
- Page 100 and 101:
Mixed Mode Survey Administration Ma
- Page 102 and 103:
Mixed Mode Survey Administration Ma
- Page 104 and 105:
Mixed Mode Survey Administration Ma
- Page 106 and 107:
Mixed Mode Survey Administration Ma
- Page 108 and 109:
100 Centers for Medicare & Medicaid
- Page 110 and 111:
Active Interactive Voice Response (
- Page 112 and 113:
Active Interactive Voice Response (
- Page 114 and 115:
Active Interactive Voice Response (
- Page 116 and 117:
Active Interactive Voice Response (
- Page 118 and 119:
Active Interactive Voice Response (
- Page 120 and 121:
Data Specifications and Coding Marc
- Page 122 and 123:
Data Specifications and Coding Marc
- Page 124 and 125:
Data Specifications and Coding Marc
- Page 126 and 127:
Data Specifications and Coding Marc
- Page 128 and 129:
Data Specifications and Coding Marc
- Page 130 and 131:
Data Specifications and Coding Marc
- Page 132 and 133:
Data Specifications and Coding Marc
- Page 134 and 135:
Data Specifications and Coding Marc
- Page 136 and 137:
Data Specifications and Coding Marc
- Page 138 and 139:
Data Specifications and Coding Marc
- Page 140 and 141:
Data Preparation and Submission Mar
- Page 142 and 143:
Data Preparation and Submission Mar
- Page 144 and 145:
Data Preparation and Submission Mar
- Page 146 and 147:
Data Preparation and Submission Mar
- Page 148 and 149:
Data Preparation and Submission Mar
- Page 150 and 151:
142 Centers for Medicare & Medicaid
- Page 152 and 153:
Oversight Activities March 2011 HCA
- Page 154 and 155:
Oversight Activities March 2011 wit
- Page 156 and 157:
148 Centers for Medicare & Medicaid
- Page 158 and 159:
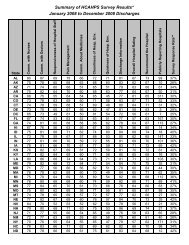
Data Reporting March 2011 Bar graph
- Page 160 and 161:
152 Centers for Medicare & Medicaid
- Page 162 and 163:
Exceptions Request/Discrepancy Repo
- Page 164 and 165:
156 Centers for Medicare & Medicaid
- Page 166 and 167:
Appendices March 2011 O. Participat
- Page 169 and 170:
HCAHPS Survey SURVEY INSTRUCTIONS
- Page 171 and 172:
15. During this hospital stay, were
- Page 173 and 174:
HCAHPS Survey SURVEY INSTRUCTIONS
- Page 175 and 176:
14. During this hospital stay, how
- Page 177 and 178:
Sample Initial Cover Letter for the
- Page 179 and 180:
Sample Follow-up Cover Letter for t
- Page 181 and 182:
OMB Paperwork Reduction Act Languag
- Page 183:
APPENDIX B HCAHPS MAIL SURVEY (Span
- Page 186 and 187:
4. Durante esta vez que estuvo en e
- Page 188 and 189:
CALIFICACIÓN GENERAL DEL HOSPITAL
- Page 190 and 191:
6 March 2011
- Page 192 and 193:
4. Durante esta vez que estuvo en e
- Page 194 and 195:
CALIFICACIÓN GENERAL DEL HOSPITAL
- Page 196 and 197:
12 March 2011
- Page 198 and 199:
14 March 2011
- Page 200 and 201:
16 March 2011
- Page 202 and 203:
18 March 2011
- Page 205 and 206:
HCAHPS 意 見 調 查 問 卷 指
- Page 207 and 208:
14. 此 次 住 院 期 間 , 醫
- Page 209 and 210:
26. 您 屬 於 哪 一 種 族 ?
- Page 211 and 212:
問 卷 指 示 ♦ 您 是 信 函
- Page 213 and 214:
12. 此 次 住 院 期 間 , 您
- Page 215 and 216:
26. 您 屬 於 哪 一 種 族 ?
- Page 217 and 218:
Sample Initial Cover Letter for the
- Page 219 and 220:
Sample Follow-Up Cover Letter for t
- Page 221 and 222:
OMB 減 低 公 文 法 案 Overvie
- Page 223:
APPENDIX D HCAHPS MAIL SURVEY (Russ
- Page 226 and 227:
4. Во время данного
- Page 228 and 229:
ОБЩИЙ РЕЙТИНГ БОЛЬ
- Page 230 and 231:
6 March 2011
- Page 232 and 233:
4. Во время данного
- Page 234 and 235:
ОБЩИЙ РЕЙТИНГ БОЛЬ
- Page 236 and 237:
12 March 2011
- Page 238 and 239:
14 March 2011
- Page 240 and 241:
16 March 2011
- Page 242 and 243:
18 March 2011
- Page 245 and 246:
THĂM DÒ Ý KIẾN HCAHPS CHỈ D
- Page 247 and 248:
14. Trong lần nằm bệnh viện
- Page 249 and 250:
THĂM DÒ Ý KIẾN HCAHPS CHỈ D
- Page 251 and 252:
15. Trong lần nằm bệnh viện
- Page 253 and 254:
Sample Initial Cover Letter for the
- Page 255 and 256:
[SAMPLED PATIENT NAME] [ADDRESS] [C
- Page 257 and 258:
OMB Paperwork Reduction Act Languag
- Page 259:
APPENDIX F Telephone Script (Englis
- Page 262 and 263:
NOTE: SEE INTERVIEWING GUIDELINES I
- Page 264 and 265:
Q2 During this hospital stay, how o
- Page 266 and 267:
Q11 How often did you get help in g
- Page 268 and 269:
Q19 During this hospital stay, did
- Page 270 and 271:
[FOR TELEPHONE INTERVIEWING, QUESTI
- Page 272 and 273:
12 Centers for Medicare & Medicaid
- Page 275 and 276:
Overview HCAHPS Telephone Script (S
- Page 277 and 278:
S1 La información que tenemos indi
- Page 279 and 280:
Q6 Durante esta vez que estuvo en e
- Page 281 and 282:
Q14 Durante esta vez que estuvo en
- Page 283 and 284:
Q21 Queremos saber la calificación
- Page 285 and 286:
Q26A ¿Es usted Blanco/a? Sí/Blan
- Page 287:
APPENDIX H IVR Script (English)
- Page 290 and 291:
NOTE: SEE INTERVIEWING GUIDELINES I
- Page 292 and 293:
Q1 During this hospital stay, how o
- Page 294 and 295:
Q9 During this hospital stay, how o
- Page 296 and 297:
Q16 Before giving you any new medic
- Page 298 and 299:
Q23_INTRO This last set of question
- Page 300 and 301:
Q27 What language do you mainly spe
- Page 303 and 304:
HCAHPS Interviewing Guidelines for
- Page 305 and 306:
• Skip patterns should be program
- Page 307:
APPENDIX J Frequently Asked Questio
- Page 310 and 311:
‣ How can I verify this survey is
- Page 312 and 313:
I understand your concern. This is
- Page 314 and 315:
6 Centers for Medicare & Medicaid S
- Page 317 and 318:
HCAHPS SAMPLE FRAME FILE LAYOUT Bel
- Page 319 and 320:
Data Element Length Value Labels an
- Page 321 and 322:
Data Element Length Value Labels an
- Page 323:
APPENDIX L Data File Structure Vers
- Page 326 and 327:
Determination of Service Line Elig
- Page 328 and 329:
Discharge Status Patient’s disch
- Page 330 and 331:
Survey Language Identify whether s
- Page 332 and 333:
Q9 “During this hospital stay, h
- Page 334 and 335:
Q22 “Would you recommend this ho
- Page 336 and 337:
12 Centers for Medicare & Medicaid
- Page 339 and 340:
07/01/2011 and forward discharges H
- Page 341 and 342:
07/01/2011 and forward discharges H
- Page 343 and 344:
07/01/2011 and forward discharges H
- Page 345 and 346:
07/01/2011 and forward discharges H
- Page 347 and 348:
07/01/2011 and forward discharges H
- Page 349 and 350:
07/01/2011 and forward discharges H
- Page 351 and 352:
07/01/2011 and forward discharges H
- Page 353 and 354:
07/01/2011 and forward discharges H
- Page 355 and 356:
07/01/2011 and forward discharges H
- Page 357 and 358:
07/01/2011 and forward discharges H
- Page 359 and 360:
07/01/2011 and forward discharges H
- Page 361 and 362:
07/01/2011 and forward discharges H
- Page 363 and 364:
07/01/2011 and forward discharges H
- Page 365 and 366:
07/01/2011 and forward discharges H
- Page 367 and 368:
07/01/2011 and forward discharges H
- Page 369 and 370:
07/01/2011 and forward discharges H
- Page 371 and 372:
07/01/2011 and forward discharges H
- Page 373 and 374:
Sample XML File Layout Without DSRS
- Page 375 and 376:
1 1 1 1 1 1 1 1 1 1 1 1 0 0 0 0 1
- Page 377 and 378:
- 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1
- Page 379 and 380:
10 1 1 - 1 1 1 1 1 1 1 1 1 1 1 1
- Page 381:
APPENDIX N Quality Assurance Plan O
- Page 384 and 385:
4. Provide and attach an HCAHPS org
- Page 386 and 387:
C. Describe the process for convert
- Page 388 and 389:
D. Describe any opportunities for i
- Page 391 and 392:
HCAHPS Participation Form For Hospi
- Page 393 and 394:
2. Organizational Survey Capacity I
- Page 395:
APPENDIX P Participation Form for H
- Page 398 and 399:
3. TYPE(S) OF MODE OF SURVEY ADMINI
- Page 400 and 401:
Hospital Name and Address (required
- Page 402 and 403:
6 Centers for Medicare & Medicaid S
- Page 405 and 406:
HCAHPS Participation Form For Surve
- Page 407 and 408:
IVR Mode of Survey Administration (
- Page 409 and 410:
If not submitting this form online
- Page 411:
APPENDIX R Exceptions Request Form
- Page 414 and 415:
II. Exception Request Please comple
- Page 417 and 418:
HCAHPS DISCREPANCY REPORT FORM Sect