Exergy saving and exergy production in municipal wastewater ...
Exergy saving and exergy production in municipal wastewater ...
Exergy saving and exergy production in municipal wastewater ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Pegah Piri TRITA-LWR Degree Project 12:14<br />
1 g biogas=43 kJ <strong>exergy</strong><br />
There have been measurements for the <strong>exergy</strong> values of some of the<br />
products (McCarthy <strong>and</strong> Rittman, 2000).<br />
The <strong>exergy</strong> content <strong>in</strong> element with lower TOC/COD values is higher<br />
<strong>and</strong> this will get decreased as the TOC/COD ratio <strong>in</strong> the element<br />
<strong>in</strong>creases (Fig. 3). Additionally, there is a correlation between the number<br />
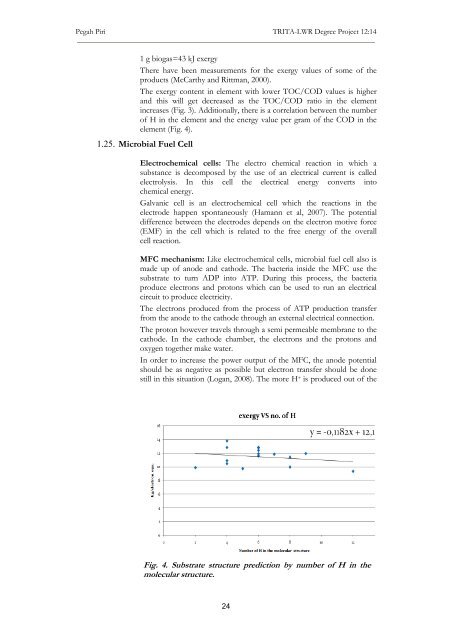
of H <strong>in</strong> the element <strong>and</strong> the energy value per gram of the COD <strong>in</strong> the<br />
element (Fig. 4).<br />
1.25. Microbial Fuel Cell<br />
Electrochemical cells: The electro chemical reaction <strong>in</strong> which a<br />
substance is decomposed by the use of an electrical current is called<br />
electrolysis. In this cell the electrical energy converts <strong>in</strong>to<br />
chemical energy.<br />
Galvanic cell is an electrochemical cell which the reactions <strong>in</strong> the<br />
electrode happen spontaneously (Hamann et al, 2007). The potential<br />
difference between the electrodes depends on the electron motive force<br />
(EMF) <strong>in</strong> the cell which is related to the free energy of the overall<br />
cell reaction.<br />
MFC mechanism: Like electrochemical cells, microbial fuel cell also is<br />
made up of anode <strong>and</strong> cathode. The bacteria <strong>in</strong>side the MFC use the<br />
substrate to turn ADP <strong>in</strong>to ATP. Dur<strong>in</strong>g this process, the bacteria<br />
produce electrons <strong>and</strong> protons which can be used to run an electrical<br />
circuit to produce electricity.<br />
The electrons produced from the process of ATP <strong>production</strong> transfer<br />
from the anode to the cathode through an external electrical connection.<br />
The proton however travels through a semi permeable membrane to the<br />
cathode. In the cathode chamber, the electrons <strong>and</strong> the protons <strong>and</strong><br />
oxygen together make water.<br />
In order to <strong>in</strong>crease the power output of the MFC, the anode potential<br />
should be as negative as possible but electron transfer should be done<br />
still <strong>in</strong> this situation (Logan, 2008). The more H + is produced out of the<br />
Fig. 4. Substrate structure prediction by number of H <strong>in</strong> the<br />
molecular structure.<br />
24