pNiFty-SEAP protocol - InvivoGen
pNiFty-SEAP protocol - InvivoGen
pNiFty-SEAP protocol - InvivoGen
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>pNiFty</strong>-<strong>SEAP</strong><br />
A TLR-signaling reporter plasmid<br />
Catalog # pnifty-seap<br />
For research use only<br />
Version # 04B03-SV<br />
PRODUCT INFORMATION<br />
Content:<br />
• 1 disk of lyophilized E. coli GT110 transformed with <strong>pNiFty</strong>-<strong>SEAP</strong>.<br />
GT110 genotype:<br />
F-, mcrA, Δ(mrr-hsdRMS-mcrBC), Ø80lacZ∆M15, ∆lacX74, recA1, endA1<br />
• 4 pouches of E. coli Fast-Media ® Amp (2 TB and 2 Agar).<br />
Storage and stability:<br />
Products are shipped at room temperature.<br />
Transformed bacteria should be stored at -20°C and are stable up to<br />
1 year.<br />
Store E. coli Fast-Media ® Amp at room temperature. Fast-Media ® pouches<br />
are stable 18 months when stored properly.<br />
Quality control:<br />
Plasmid construct has been confirmed by restriction analysis and<br />
sequencing.<br />
Bacteria have been lyophilized, and their viability upon resuspension has<br />
been verified.<br />
GENERAL PRODUCT USE<br />
Toll-Like receptors (TLRs) play a critical role in early innate immunity to<br />
invading pathogens by sensing microorganisms 1-4 . These evolutionary<br />
conserved receptors, homologues of the Drosophila Toll gene, recognize<br />
highly conserved structural motifs only expressed by microbial<br />
pathogens, called pathogen-associated microbial patterns (PA M P s ) .<br />
PAMPs include various bacterial cell wall components such as<br />
lipopolysaccharides (LPS), peptidoglycans and lipopeptides, as well as<br />
flagellin, bacterial DNA and viral double-stranded RNA. Stimulation of<br />
TLRs by PAMPs initiates a signaling cascade that involves a number of<br />
proteins, such as MyD88 and IRAK.<br />
TLR signaling leads to the translocation of NF-κB, a transcription factor<br />
implicated in the activation of numerous genes such as the endothelial<br />
cell-leukocyte adhesion molecule (ELAM-1, E-selectin) gene 5 . To<br />
monitor the induction of TLR signaling in a simple and efficient manner,<br />
<strong>InvivoGen</strong> has designed <strong>pNiFty</strong>, a family of plasmids carrying a reporter<br />
gene, encoding secreted alkaline phosphatase (<strong>SEAP</strong>) or luciferase, which<br />
expression is controlled by an NF-κB-inducible ELAM-1 composite<br />
promoter.<br />
<strong>pNiFty</strong> is only selectable in E. coli with ampicillin. The plasmid can be<br />
used to transiently transfect TLR-expressing cells, such as dendritic cells,<br />
or cells transfected with a pUNO-TLR or pDUO-TLR plasmid.<br />
PLASMID FEATURES<br />
• NF-κB5-ELAM is an engineered ELAM promoter combining five NFκB<br />
sites (GGGGACTTTCC) with the proximal ELAM promoter 5 . In the<br />
absence of NF-κB, the promoter displays no or very little activity.<br />
Induction by NF-κB activates the promoter resulting in the expression of<br />
the <strong>SEAP</strong> gene at levels similar to those obtained with a strong promoter<br />
such as CMV or EF1.<br />
• Ori is a minimal E. coli origin of replication with the same activity as<br />
the longer Ori.<br />
• Amp: The ampicillin resistance gene allows amplification of the<br />
plasmid in bacteria.<br />
• <strong>SEAP</strong> is a secreted form of human embryonic alkaline phosphatase.<br />
Unlike endogenous alkaline phosphatases, <strong>SEAP</strong> is extremely heat stable<br />
and resistant to the inhibitor L-homoarginine. It catalyses the hydrolysis<br />
of pNitrophenyl phosphate (pNpp) producing a yellow end product.<br />
<strong>SEAP</strong> expression can be readily quantified by collecting samples of<br />
culture medium and measuring the hydrolysis of pNpp with a<br />
spectrophometer at 405 nm.<br />
• SV40 pAn: The Simian Virus 40 late polyadenylation signal enables<br />
efficient cleavage and polyadenylation reactions resulting in high levels<br />
of steady-state mRNA.<br />
References<br />
1. Aderem A. & Ulevitch R.J.(2000). Nature, 406(6797):782-7<br />
2. Underhill D. & Ozinsky A. (2002). Curr Opin Immunol 14(1): 103-110.<br />
3. Takeuchi O.& Akira S.(2002). Microbes Infect. 4(9): 887-895.<br />
4. Akira S. (2003). Curr Opin Immunol 15: 1-7.<br />
5. Schindler U. & Baichwal V.R. (1994). Mol Cell Biol. 14(9):5820-31<br />
METHODS<br />
Growth of <strong>pNiFty</strong>-transformed bacteria:<br />
Use sterile conditions to do the following:<br />
1- Resuspend the lyophilized E. coli transformed cells by adding 1 ml of<br />
LB medium in the tube containing the disk. Let sit for 5 minutes. Mix<br />
gently by inverting the tube several times.<br />
2- Streak bacteria taken from this suspension on a ampicillin LB agar<br />
plate prepared with the E. coli Fast-Media ® Amp agar provided (see<br />
below).<br />
3- Place the plate in an incubator at 37˚C overnight.<br />
4- Isolate a single colony and grow the bacteria in TB supplemented with<br />
ampicillin using the Fast-Media ® Amp liquid provided (see below).<br />
5- Extract the <strong>pNiFty</strong> plasmid DNA using the method of your choice.<br />
Selection of bacteria with E. coli Fast-Media Amp:<br />
E. coli Fast-Media ® Amp is a fast and convenient way to prepare liquid<br />
and solid media for bacterial culture by using only a microwave.<br />
1- Pour the contents of a pouch into a clean borosilicate glass bottle or<br />
flask.<br />
2- Add 200 ml of distilled water to the flask.<br />
3- Heat in a microwave on MEDIUM power setting (about 400 Watts),<br />
until bubbles start appearing (approximately 3 minutes). Do not heat a<br />
closed container. Do not autoclave Fast-Media ® .<br />
4- Swirl gently to mix the preparation. Be careful, the bottle and media<br />
are hot, use heatproof pads or gloves and care when handling.<br />
5- Reheat the media for 30 seconds and gently swirl again. Repeat as<br />
necessary to completely dissolve the powder into solution. But be careful<br />
to avoid overboiling and volume loss.<br />
6- Let agar medium cool to 45˚C before pouring plates. Let liquid media<br />
cool to 37˚C before seeding bacteria.<br />
Note: Do not reheat solidified Fast-Media ® as the antibiotic will be<br />
permanently destroyed by the procedure.<br />
TECHNICAL SUPPORT<br />
Toll free (US): 888-457-5873<br />
Outside US: (+1) 858-457-5873<br />
E-mail: info@invivogen.com<br />
Website: www.invivogen.com<br />
3950 Sorrento Valley Blvd. Suite A<br />
San Diego, CA 92121 - USA
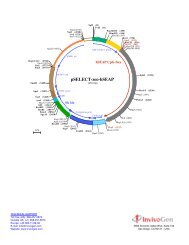
SgfI (12) EcoRI (17)<br />
PstI (99)<br />
NotI (4269)<br />
PvuII (232)<br />
NF-kB 5x<br />
ELAM<br />
PstI (434)<br />
PvuII (505)<br />
BamHI (512)<br />
AseI (3220)<br />
Amp<br />
<strong>pNiFty</strong>-<strong>SEAP</strong><br />
(4431 bp)<br />
<strong>SEAP</strong><br />
pMB1 ori<br />
SV40 pAn<br />
NheI (1850)<br />
HpaI (2015)<br />
SwaI (2115)<br />
125
1<br />
101<br />
SgfI (12) EcoRI (17) PstI (99)<br />
GGATCTGCGATCGCTGAATTCTGGGGACTTTCCACTGGGGACTTTCCACTGGGGACTTTCCACTGGGGACTTTCCACTGGGGACTTTCCACTCCTGCAGC<br />
AGTGGATATTCCCAGAAAACTTTTTGGATGCAGTTGGGGATTTCCTCTTTACTGGATGTGGACAATATCCTCCTATTATTCACAGGAAGCAATCCCTCCT<br />
PvuII (232)<br />
201 ATAAAAGGGCCTCAGAGGAAGTAGTGTTCAGCTGTTCTTGGCTGACTTCACATCAAAGCTCCTATACTGACCTGAGACAGAGCCATGATTCTGGGGCCCT<br />
1 MetI leLeuGlyProC<br />
301 GCATGCTGCTGCTGCTGCTGCTGCTGGGCCTGAGGCTACAGCTCTCCCTGGGCATCATCCCAGTTGAGGAGGAGAACCCGGACTTCTGGAACCGCGAGGC<br />
6 ysMetLeuLeuLeuLeuLeuLeuLeuGlyLeuArgLeuGlnLeuSerLeuGlyI leI leProValGluGluGluAsnProAspPheTrpAsnArgGluAl<br />
PstI (434)<br />
401 AGCCGAGGCCCTGGGTGCCGCCAAGAAGCTGCAGCCTGCACAGACAGCCGCCAAGAACCTCATCATCTTCCTGGGCGATGGGATGGGGGTGTCTACGGTG<br />
39 aAlaGluAlaLeuGlyAlaAlaLysLysLeuGlnProAlaGlnThrAlaAlaLysAsnLeuI leI lePheLeuGlyAspGlyMetGlyValSerThrVal<br />
PvuII (505) BamHI (512)<br />
501 ACAGCTGCCAGGATCCTAAAAGGGCAGAAGAAGGACAAACTGGGGCCTGAGATACCCCTGGCTATGGACCGCTTCCCATATGTGGCTCTGTCCAAGACAT<br />
73 ThrAlaAlaArgI leLeuLysGlyGlnLysLysAspLysLeuGlyProGluI leProLeuAlaMetAspArgPheProTyrValAlaLeuSerLysThrT<br />
601 ACAATGTAGACAAACATGTGCCAGACAGTGGAGCCACAGCCACGGCCTACCTGTGCGGGGTCAAGGGCAACTTCCAGACCATTGGCTTGAGTGCAGCCGC<br />
106 yrAsnValAspLysHisValProAspSerGlyAlaThrAlaThrAlaTyrLeuCysGlyValLysGlyAsnPheGlnThrI leGlyLeuSerAlaAlaAl<br />
701 CCGCTTTAACCAGTGCAACACGACACGCGGCAACGAGGTCATCTCCGTGATGAATCGGGCCAAGAAAGCAGGGAAGTCAGTGGGAGTGGTAACCACCACA<br />
139 aArgPheAsnGlnCysAsnThrThrArgGlyAsnGluVal I leSerValMetAsnArgAlaLysLysAlaGlyLysSerValGlyValValThrThrThr<br />
801 CGAGTGCAGCACGCCTCGCCAGCCGGCACCTACGCCCACACGGTGAACCGCAACTGGTACTCGGACGCCGACGTGCCTGCCTCGGCCCGCCAGGAGGGGT<br />
173 ArgValGlnHisAlaSerProAlaGlyThrTyrAlaHisThrValAsnArgAsnTrpTyrSerAspAlaAspValProAlaSerAlaArgGlnGluGlyC<br />
901 GCCAGGACATCGCTACGCAGCTCATCTCCAACATGGACATTGATGTGATCCTGGGTGGAGGCCGAAAGTACATGTTTCGCATGGGAACCCCAGACCCTGA<br />
206 ysGlnAspI leAlaThrGlnLeuI leSerAsnMetAspI leAspVal I leLeuGlyGlyGlyArgLysTyrMetPheArgMetGlyThrProAspProGl<br />
1001 GTACCCAGATGACTACAGCCAAGGTGGGACCAGGCTGGACGGGAAGAATCTGGTGCAGGAATGGCTGGCGAAGCGCCAGGGTGCCCGGTATGTGTGGAAC<br />
239 uTyrProAspAspTyrSerGlnGlyGlyThrArgLeuAspGlyLysAsnLeuValGlnGluTrpLeuAlaLysArgGlnGlyAlaArgTyrValTrpAsn<br />
1101 CGCACTGAGCTCATGCAGGCTTCCCTGGACCCGTCTGTGACCCATCTCATGGGTCTCTTTGAGCCTGGAGACATGAAATACGAGATCCACCGAGACTCCA<br />
273 ArgThrGluLeuMetGlnAlaSerLeuAspProSerValThrHisLeuMetGlyLeuPheGluProGlyAspMetLysTyrGluI leHisArgAspSerT<br />
1201 CACTGGACCCCTCCCTGATGGAGATGACAGAGGCTGCCCTGCGCCTGCTGAGCAGGAACCCCCGCGGCTTCTTCCTCTTCGTGGAGGGTGGTCGCATCGA<br />
306 hrLeuAspProSerLeuMetGluMetThrGluAlaAlaLeuArgLeuLeuSerArgAsnProArgGlyPhePheLeuPheValGluGlyGlyArgI leAs<br />
1301 CCACGGTCATCACGAAAGCAGGGCTTACCGGGCACTGACTGAGACGATCATGTTCGACGACGCCATTGAGAGGGCGGGCCAGCTCACCAGCGAGGAGGAC<br />
339 pHisGlyHisHisGluSerArgAlaTyrArgAlaLeuThrGluThrI leMetPheAspAspAlaI leGluArgAlaGlyGlnLeuThrSerGluGluAsp<br />
1401 ACGCTGAGCCTCGTCACTGCCGACCACTCCCACGTCTTCTCCTTCGGAGGCTACCCCCTGCGAGGGAGCTCCATCTTCGGGCTGGCCCCTGGCAAGGCCC<br />
373 ThrLeuSerLeuValThrAlaAspHisSerHisValPheSerPheGlyGlyTyrProLeuArgGlySerSerI lePheGlyLeuAlaProGlyLysAlaA<br />
1501 GGGACAGGAAGGCCTACACGGTCCTCCTATACGGAAACGGTCCAGGCTATGTGCTCAAGGACGGCGCCCGGCCGGATGTTACCGAGAGCGAGAGCGGGAG<br />
406 rgAspArgLysAlaTyrThrValLeuLeuTyrGlyAsnGlyProGlyTyrValLeuLysAspGlyAlaArgProAspValThrGluSerGluSerGlySe<br />
1601 CCCCGAGTATCGGCAGCAGTCAGCAGTGCCCCTGGACGAAGAGACCCACGCAGGCGAGGACGTGGCGGTGTTCGCGCGCGGCCCGCAGGCGCACCTGGTT<br />
439 rProGluTyrArgGlnGlnSerAlaValProLeuAspGluGluThrHisAlaGlyGluAspValAlaValPheAlaArgGlyProGlnAlaHisLeuVal<br />
1701 CACGGCGTGCAGGAGCAGACCTTCATAGCGCACGTCATGGCCTTCGCCGCCTGCCTGGAGCCCTACACCGCCTGCGACCTGGCGCCCCCCGCCGGCACCA<br />
473 HisGlyValGlnGluGlnThrPheI leAlaHisValMetAlaPheAlaAlaCysLeuGluProTyrThrAlaCysAspLeuAlaProProAlaGlyThrT<br />
NheI (1850)<br />
1801 CCGACGCCGCGCACCCGGGGCGGTCCCGGTCCAAGCGTCTGGATTGAAGCTAGCTCGACATGATAAGATACATTGATGAGTTTGGACAAACCACAACTAG<br />
506 hrAspAlaAlaHisProGlyArgSerArgSerLysArgLeuAsp•••<br />
1901 AATGCAGTGAAAAAAATGCTTTATTTGTGAAATTTGTGATGCTATTGCTTTATTTGTGAAATTTGTGATGCTATTGCTTTATTTGTAACCATTATAAGCT<br />
2001<br />
2101<br />
2201<br />
2301<br />
2401<br />
2501<br />
2601<br />
2701<br />
2801<br />
2901<br />
3001<br />
278<br />
3101<br />
244<br />
3201<br />
211<br />
3301<br />
178<br />
HpaI (2015)<br />
GCAATAAACAAGTTAACAACAACAATTGCATTCATTTTATGTTTCAGGTTCAGGGGGAGGTGTGGGAGGTTTTTTAAAGCAAGTAAAACCTCTACAAATG<br />
SwaI (2115)<br />
TGGTAGATCCATTTAAATGTTAATTAAAAACCCGCTTCGGCGGGTTTTTTTATGCATGTGAGCAAAAGGCCAGCAAAAGGCCAGGAACCGTAAAAAGGCC<br />
GCGTTGCTGGCGTTTTTCCATAGGCTCCGCCCCCCTGACGAGCATCACAAAAATCGACGCTCAAGTCAGAGGTGGCGAAACCCGACAGGACTATAAAGAT<br />
ACCAGGCGTTTCCCCCTGGAAGCTCCCTCGTGCGCTCTCCTGTTCCGACCCTGCCGCTTACCGGATACCTGTCCGCCTTTCTCCCTTCGGGAAGCGTGGC<br />
GCTTTCTCATAGCTCACGCTGTAGGTATCTCAGTTCGGTGTAGGTCGTTCGCTCCAAGCTGGGCTGTGTGCACGAACCCCCCGTTCAGCCCGACCGCTGC<br />
GCCTTATCCGGTAACTATCGTCTTGAGTCCAACCCGGTAAGACACGACTTATCGCCACTGGCAGCAGCCACTGGTAACAGGATTAGCAGAGCGAGGTATG<br />
TAGGCGGTGCTACAGAGTTCTTGAAGTGGTGGCCTAACTACGGCTACACTAGAAGAACAGTATTTGGTATCTGCGCTCTGCTGAAGCCAGTTACCTTCGG<br />
AAAAAGAGTTGGTAGCTCTTGATCCGGCAAACAAACCACCGCTGGTAGCGGTGGTTTTTTTGTTTGCAAGCAGCAGATTACGCGCAGAAAAAAAGGATCT<br />
CAAGAAGATCCTTTGATCTTTTCTACGGGGTCTGACGCTCAGTGGAACGAAAACTCACGTTAAGGGATTTTGGTCATGAGATTATCAAAAAGGATCTTCA<br />
CCTAGATCCTTTTAAATTAAAAATGAAGTTTTAAATCAATCTAAAGTATATATGAGTAAACTTGGTCTGACAGTTACCAATGCTTAATCAGTGAGGCACC<br />
287 •••TrpHisLysI leLeuSerAlaGly<br />
TATCTCAGCGATCTGTCTATTTCGTTCATCCATAGTTGCCTGACTCCCCGTCGTGTAGATAACTACGATACGGGAGGGCTTACCATCTGGCCCCAGTGCT<br />
I leGluAlaI leGlnArgAsnArgGluAspMetThrAlaGlnSerGlyThrThrTyrI leValVal I leArgSerProLysGlyAspProGlyLeuAlaA<br />
GCAATGATACCGCGAGACCCACGCTCACCGGCTCCAGATTTATCAGCAATAAACCAGCCAGCCGGAAGGGCCGAGCGCAGAAGTGGTCCTGCAACTTTAT<br />
laI leI leGlyArgSerGlyArgGluGlyAlaGlySerLysAspAlaI lePheTrpGlyAlaProLeuAlaSerArgLeuLeuProGlyAlaValLysAs<br />
AseI (3220)<br />
CCGCCTCCATCCAGTCTATTAATTGTTGCCGGGAAGCTAGAGTAAGTAGTTCGCCAGTTAATAGTTTGCGCAACGTTGTTGCCATTGCTACAGGCATCGT<br />
pAlaGluMetTrpAspI leLeuGlnGlnArgSerAlaLeuThrLeuLeuGluGlyThrLeuLeuLysArgLeuThrThrAlaMetAlaValProMetThr<br />
GGTGTCACGCTCGTCGTTTGGTATGGCTTCATTCAGCTCCGGTTCCCAACGATCAAGGCGAGTTACATGATCCCCCATGTTGTGCAAAAAAGCGGTTAGC<br />
ThrAspArgGluAspAsnProI leAlaGluAsnLeuGluProGluTrpArgAspLeuArgThrValHisAspGlyMetAsnHisLeuPheAlaThrLeuG
3401 TCCTTCGGTCCTCCGATCGTTGTCAGAAGTAAGTTGGCCGCAGTGTTATCACTCATGGTTATGGCAGCACTGCATAATTCTCTTACTGTCATGCCATCCG<br />
144 luLysProGlyGlyI leThrThrLeuLeuLeuAsnAlaAlaThrAsnAspSerMetThrI leAlaAlaSerCysLeuGluArgValThrMetGlyAspTh<br />
3501 TAAGATGCTTTTCTGTGACTGGTGAGTACTCAACCAAGTCATTCTGAGAATAGTGTATGCGGCGACCGAGTTGCTCTTGCCCGGCGTCAATACGGGATAA<br />
111 rLeuHisLysGluThrValProSerTyrGluValLeuAspAsnGlnSerTyrHisI leArgArgGlyLeuGlnGluGlnGlyAlaAspI leArgSerLeu<br />
3601 TACCGCGCCACATAGCAGAACTTTAAAAGTGCTCATCATTGGAAAACGTTCTTCGGGGCGAAAACTCTCAAGGATCTTACCGCTGTTGAGATCCAGTTCG<br />
78 ValAlaGlyCysLeuLeuValLysPheThrSerMetMetProPheArgGluGluProArgPheSerGluLeuI leLysGlySerAsnLeuAspLeuGluI<br />
3701 ATGTAACCCACTCGTGCACCCAACTGATCTTCAGCATCTTTTACTTTCACCAGCGTTTCTGGGTGAGCAAAAACAGGAAGGCAAAATGCCGCAAAAAAGG<br />
44 leTyrGlyValArgAlaGlyLeuGlnAspGluAlaAspLysValLysValLeuThrGluProHisAlaPheValProLeuCysPheAlaAlaPhePhePr<br />
3801 GAATAAGGGCGACACGGAAATGTTGAATACTCATACTCTTCCTTTTTCAATATTATTGAAGCATTTATCAGGGTTATTGTCTCATGAGCGGATACATATT<br />
11 oI leLeuAlaValArgPheHisGlnI leSerMet<br />
3901 TGAATGTATTTAGAAAAATAAACAAATAGGGGTTCCGCGCACATTTCCCCGAAAAGTGCCACCTGACGTCTAAGAAACCATTATTATCATGACATTAACC<br />
4001 TATAAAAATAGGCGTATCACGAGGCCCTTTCGTCTCGCGCGTTTCGGTGATGACGGTGAAAACCTCTGACACATGCAGCTCCCGGAGACGGTCACAGCTT<br />
4101 GTCTGTAAGCGGATGCCGGGAGCAGACAAGCCCGTCAGGGCGCGTCAGCGGGTGTTGGCGGGTGTCGGGGCTGGCTTAACTATGCGGCATCAGAGCAGAT<br />
NotI (4269)<br />
4201 TGTACTGAGAGTGCACCATATGGATCTCGATAACAAAAAACCCCGCCCCGGCGGGGTTTTTTGTTAGCGGCCGCAATAAAATATCTTTATTTTCATTACA<br />
4301 TCTGTGTGTTGGTTTTTTGTGTGAATCGTAACTAACATACGCTCTCCATCAAAACAAAACGAAACAAAACAAACTAGCAAAATAGGCTGTCCCCAGTGCA<br />
4401 AGTGCAGGTGCCAGAACATTTCTCTATCGAA