Heavy Metals Uptake by Dried Caulerpa lentillifera - The Joint ...
Heavy Metals Uptake by Dried Caulerpa lentillifera - The Joint ...
Heavy Metals Uptake by Dried Caulerpa lentillifera - The Joint ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>The</strong> 2 nd <strong>Joint</strong> International Conference on “Sustainable Energy and Environment (SEE 2006)”<br />
E-043 (P) 21-23 November 2006, Bangkok, Thailand<br />
V ( Ci − C<br />
f<br />
)<br />
q = (1)<br />
m<br />
where q is amount of metal uptake per unit mass of biomass (mol kg -1 ), C i is initial concentration of heavy metal (mol m -3 ), C f is<br />
final concentration of heavy metal (mol m -3 ), V is volume of the solution (m 3 ), m is dry mass of the algae (kg).<br />
3. RESULTS AND DISCUSSION<br />
3.1 Effect of initial metal concentration on biosorption<br />
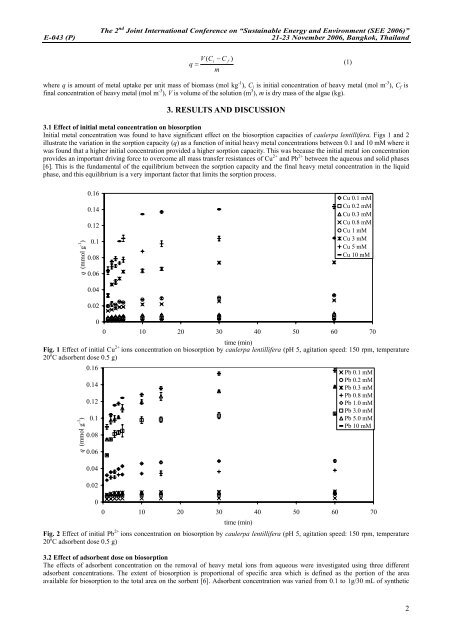
Initial metal concentration was found to have significant effect on the biosorption capacities of caulerpa <strong>lentillifera</strong>. Figs 1 and 2<br />
illustrate the variation in the sorption capacity (q) as a function of initial heavy metal concentrations between 0.1 and 10 mM where it<br />
was found that a higher initial concentration provided a higher sorption capacity. This was because the initial metal ion concentration<br />
provides an important driving force to overcome all mass transfer resistances of Cu 2+ and Pb 2+ between the aqueous and solid phases<br />
[6]. This is the fundamental of the equilibrium between the sorption capacity and the final heavy metal concentration in the liquid<br />
phase, and this equilibrium is a very important factor that limits the sorption process.<br />
q (mmol g -1 )<br />
0.16<br />
0.14<br />
0.12<br />
0.1<br />
0.08<br />
0.06<br />
Cu 0.1 mM<br />
Cu 0.2 mM<br />
Cu 0.3 mM<br />
Cu 0.8 mM<br />
Cu 1 mM<br />
Cu 3 mM<br />
Cu 5 mM<br />
Cu 10 mM<br />
0.04<br />
0.02<br />
0<br />
0 10 20 30 40 50 60 70<br />
time (min)<br />
Fig. 1 Effect of initial Cu 2+ ions concentration on biosorption <strong>by</strong> caulerpa <strong>lentillifera</strong> (pH 5, agitation speed: 150 rpm, temperature<br />
20 o C adsorbent dose 0.5 g)<br />
q (mmol g -1 )<br />
0.16<br />
0.14<br />
0.12<br />
0.1<br />
0.08<br />
0.06<br />
0.04<br />
0.02<br />
0<br />
Pb 0.1 mM<br />
Pb 0.2 mM<br />
Pb 0.3 mM<br />
Pb 0.8 mM<br />
Pb 1.0 mM<br />
Pb 3.0 mM<br />
Pb 5.0 mM<br />
Pb 10 mM<br />
0 10 20 30 40 50 60 70<br />
time (min)<br />
Fig. 2 Effect of initial Pb 2+ ions concentration on biosorption <strong>by</strong> caulerpa <strong>lentillifera</strong> (pH 5, agitation speed: 150 rpm, temperature<br />
20 o C adsorbent dose 0.5 g)<br />
3.2 Effect of adsorbent dose on biosorption<br />
<strong>The</strong> effects of adsorbent concentration on the removal of heavy metal ions from aqueous were investigated using three different<br />
adsorbent concentrations. <strong>The</strong> extent of biosorption is proportional of specific area which is defined as the portion of the area<br />
available for biosorption to the total area on the sorbent [6]. Adsorbent concentration was varied from 0.1 to 1g/30 mL of synthetic<br />
2