Mass Transfer Between a Sphere and an Unbounded Fluid ( )
Mass Transfer Between a Sphere and an Unbounded Fluid ( )
Mass Transfer Between a Sphere and an Unbounded Fluid ( )
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Mass</strong> <strong>Tr<strong>an</strong>sfer</strong> <strong>Between</strong> a <strong>Sphere</strong> <strong><strong>an</strong>d</strong> <strong>an</strong> <strong>Unbounded</strong> <strong>Fluid</strong><br />
R. Sh<strong>an</strong>kar Subram<strong>an</strong>i<strong>an</strong><br />
Department of Chemical <strong><strong>an</strong>d</strong> Biomolecular Engineering<br />
Clarkson University<br />
When a single-component liquid drop evaporates into air, or when a solid, modeled as a singlecomponent<br />
sphere, dissolves in a liquid or sublimes into a gas, we c<strong>an</strong> construct a simple model<br />
of the diffusive tr<strong>an</strong>sport that occurs between the object <strong><strong>an</strong>d</strong> the surrounding fluid. The model<br />
c<strong>an</strong> help us calculate the rate of mass tr<strong>an</strong>sfer, <strong><strong>an</strong>d</strong> eventually the rate of ch<strong>an</strong>ge of the radius of<br />
the sphere with time.<br />
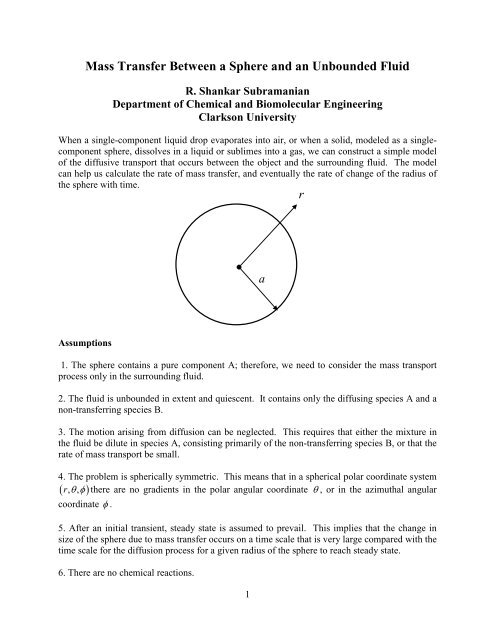
r<br />
a<br />
Assumptions<br />
1. The sphere contains a pure component A; therefore, we need to consider the mass tr<strong>an</strong>sport<br />
process only in the surrounding fluid.<br />
2. The fluid is unbounded in extent <strong><strong>an</strong>d</strong> quiescent. It contains only the diffusing species A <strong><strong>an</strong>d</strong> a<br />
non-tr<strong>an</strong>sferring species B.<br />
3. The motion arising from diffusion c<strong>an</strong> be neglected. This requires that either the mixture in<br />
the fluid be dilute in species A, consisting primarily of the non-tr<strong>an</strong>sferring species B, or that the<br />
rate of mass tr<strong>an</strong>sport be small.<br />
4. The problem is spherically symmetric. This me<strong>an</strong>s that in a spherical polar coordinate system<br />
( r, θφ , ) there are no gradients in the polar <strong>an</strong>gular coordinate θ , or in the azimuthal <strong>an</strong>gular<br />
coordinate φ .<br />
5. After <strong>an</strong> initial tr<strong>an</strong>sient, steady state is assumed to prevail. This implies that the ch<strong>an</strong>ge in<br />
size of the sphere due to mass tr<strong>an</strong>sfer occurs on a time scale that is very large compared with the<br />
time scale for the diffusion process for a given radius of the sphere to reach steady state.<br />
6. There are no chemical reactions.<br />
1
Because there are no gradients in the θ <strong><strong>an</strong>d</strong> φ directions, <strong><strong>an</strong>d</strong> there is no time-dependence, the<br />
flux N<br />
Ar<br />
depends only on r . At steady state the rate at which species A enters the spherical<br />
shell shown at location r must equal the rate at which species A leaves the shell at r+∆ r .<br />
r<br />
∆r<br />
a<br />
Using the st<strong><strong>an</strong>d</strong>ard symbol for the molar flux of A at these two locations, we c<strong>an</strong> write the steady<br />
state conservation of mass statement as<br />
2<br />
( ) π ( ) ( )<br />
πr N r − r+∆ r N r+∆ r =<br />
2<br />
4<br />
A<br />
4 0<br />
r<br />
Ar<br />
Dividing through by the factor 4π∆ r , <strong><strong>an</strong>d</strong> rearr<strong>an</strong>ging yields<br />
( r r) 2 N ( r r) r 2 N ( r)<br />
+∆<br />
A<br />
+∆ − 0<br />
r<br />
A<br />
=<br />
r<br />
∆r<br />
Taking the limit as ∆r<br />
→ 0 leads to the ordinary differential equation<br />
d<br />
rNAr<br />
dr<br />
2<br />
( )<br />
= 0<br />
We c<strong>an</strong> integrate this immediately to obtain<br />
2<br />
rNAr<br />
C1<br />
= C1<br />
, which c<strong>an</strong> be recast as NAr<br />
( r) =<br />
2<br />
r<br />
where C<br />
1<br />
is <strong>an</strong> arbitrary const<strong>an</strong>t of integration. Now, we proceed to use Fick’s law.<br />
dx<br />
( )<br />
A<br />
NAr = xA NAr + NBr − cDAB<br />
dr<br />
2
The first term in the right side corresponds to convective tr<strong>an</strong>sport, which c<strong>an</strong> be neglected in<br />
this problem because of assumptions 2 <strong><strong>an</strong>d</strong> 3. Thus, we obtain the following first order ordinary<br />
differential equation for the mole fraction of species A in the fluid.<br />
dxA<br />
C1<br />
1<br />
= −<br />
2<br />
dr c D r<br />
AB<br />
Integration of this equation is straightforward, <strong><strong>an</strong>d</strong> leads to the following solution.<br />
C1<br />
1<br />
xA<br />
( r) = + C<br />
cD r<br />
AB<br />
2<br />
There are two arbitrary const<strong>an</strong>ts that need to be evaluated. Therefore, we must write two<br />
boundary conditions. At the surface of the sphere, we c<strong>an</strong> assume equilibrium to prevail<br />
between the two phases. For example, if species A is evaporating into a gas, the partial pressure<br />
of species A in the gas phase at the interface c<strong>an</strong> be assumed to be equal to its equilibrium vapor<br />
pressure at the prevailing temperature. If the gas mixture is assumed ideal, then the mole<br />
fraction of species A in the gas phase at the interface is the ratio between this equilibrium vapor<br />
pressure of A <strong><strong>an</strong>d</strong> the prevailing total pressure in the gas phase. In non-ideal cases, a<br />
corresponding result c<strong>an</strong> be used to obtain the equilibrium mole fraction of species A in the gas<br />
phase at the interface. Likewise, for a solid dissolving in a liquid, or subliming into a gas, the<br />
equilibrium mole fraction of species A in the fluid at the interface c<strong>an</strong> be obtained.<br />
A<br />
( ) =<br />
A1<br />
x a x<br />
Far from the sphere, we c<strong>an</strong> assume the composition to approach that in the fluid in the absence<br />
of the sphere. Thus,<br />
xA<br />
( ∞ ) = 0<br />
Application of these two boundary conditions permits us to evaluate the const<strong>an</strong>ts C<br />
1<br />
<strong><strong>an</strong>d</strong> C<br />
2<br />
as<br />
C = cD ax<br />
C<br />
2<br />
= 0<br />
1 AB A1<br />
Substituting these results in the solution leads to the following result for the radial distribution of<br />
the mole fraction of species A in the fluid.<br />
( )<br />
xA<br />
r a<br />
=<br />
x r<br />
A1<br />
The flux of species A is given by<br />
3
1<br />
r<br />
( ) =<br />
1 2<br />
N r cD ax<br />
Ar AB A<br />
so that the molar rate of mass tr<strong>an</strong>sfer at the surface of the sphere c<strong>an</strong> be written as<br />
( )<br />
W = πa N a = π c D a x = π D ac<br />
2<br />
A<br />
4<br />
Ar<br />
4<br />
AB A1 4<br />
AB A1<br />
where we have used the fact that the product cxA<br />
1<br />
= cA<br />
1, the molar concentration of A in the<br />
fluid at the interface. Assuming that the molar rate of tr<strong>an</strong>sport is relatively small, we c<strong>an</strong> use a<br />
mass bal<strong>an</strong>ce on the sphere to deduce the rate of ch<strong>an</strong>ge of its size with time. Let the molecular<br />
weight of A be M , <strong><strong>an</strong>d</strong> the density of the sphere be ρ . Then, we c<strong>an</strong> write<br />
A<br />
d ⎛4<br />
da<br />
dt 3<br />
π ρ ⎞<br />
⎜ ⎟ = π ρ = −<br />
⎝ ⎠ dt<br />
π<br />
3 2<br />
a 4 a 4 DAB MA acA<br />
1<br />
which leads to a differential equation for the time-dependence of the radius of the sphere.<br />
da<br />
a dt<br />
= −<br />
DAB M<br />
A<br />
cA1<br />
ρ<br />
If the radius at time zero is a<br />
0<br />
, then the solution c<strong>an</strong> be written as<br />
2 2 2 D<br />
1<br />
( )<br />
AB<br />
M A<br />
c<br />
= A<br />
0<br />
−<br />
a t a t<br />
ρ<br />
The Quasi-Steady State Assumption<br />
Note that we assumed steady state to prevail in the diffusion problem, which, strictly speaking,<br />
requires the size of the sphere to remain unch<strong>an</strong>ged. As stated in assumption 5, this only requires<br />
that the time scale over which the sphere ch<strong>an</strong>ges appreciably in size be large compared with the<br />
time scale over which the diffusion process around a sphere of const<strong>an</strong>t size reaches steady state.<br />
Then, the rate of mass tr<strong>an</strong>sfer from the sphere to the fluid c<strong>an</strong> actually be used to calculate the<br />
time evolution of the size of the sphere. This type of assumption is called a quasi-steady state<br />
assumption. We c<strong>an</strong> make a judgment about whether it is a good assumption in a given situation<br />
by comparing these two time scales. The time needed for the diffusion process around a sphere<br />
2<br />
of radius a<br />
0<br />
to reach steady state is approximately of the same order of magnitude as a / 0<br />
D<br />
AB<br />
.<br />
By estimating the time it takes for the sphere to completely dissolve in the fluid, we c<strong>an</strong> get <strong>an</strong><br />
idea about the time scale for the size to ch<strong>an</strong>ge appreciably. From the equation for the radiustime<br />
history of the sphere, this time scale is found to be of the order of magnitude of<br />
2<br />
ρa0<br />
, where we have discarded the factor 2, because this is only <strong>an</strong> order of magnitude<br />
D M c<br />
AB A A1<br />
4
estimate. Using these results, the following estimate c<strong>an</strong> be obtained for the ratio of the two<br />
time scales.<br />
Time for diffusion process to attain steady state a / D M c<br />
= =<br />
Time for sphere to ch<strong>an</strong>ge in size appreciably ρa / D M c<br />
2<br />
0 AB A A1<br />
2<br />
0 ( AB A A1)<br />
ρ<br />
Therefore, in this problem, assumption 5 would be valid when the dimensionless group<br />
M c ρ .<br />
( A A1 ) / 1<br />
The quasi-steady state assumption is invoked commonly in problems where there are two very<br />
different time scales involved. For example, we c<strong>an</strong> use the quasi-steady assumption in the<br />
problem of calculating the rate of ch<strong>an</strong>ge of the height of liquid in a large storage t<strong>an</strong>k through a<br />
small pipe at the bottom. To calculate the velocity of flow out of the pipe <strong><strong>an</strong>d</strong> therefore the<br />
volumetric flow rate, we c<strong>an</strong> assume the level of the fluid in the t<strong>an</strong>k to remain const<strong>an</strong>t. After<br />
obtaining such a volumetric flow rate from a steady-state model, it c<strong>an</strong> be used in <strong>an</strong> unsteady<br />
mass bal<strong>an</strong>ce on the contents of the t<strong>an</strong>k to calculate the rate of ch<strong>an</strong>ge of height of the liquid in<br />
the t<strong>an</strong>k.<br />
5