You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Teacher Notes: Build - A - Molecule Activity<br />
Post lab Discussion<br />
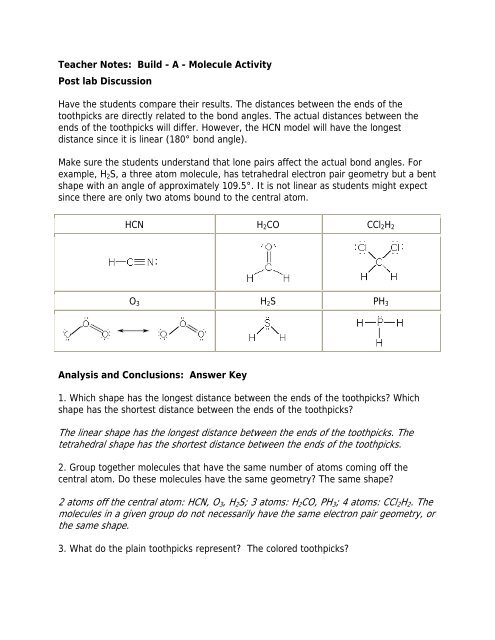
Have the students compare their results. The distances between the ends of the<br />
toothpicks are directly related to the bond angles. The actual distances between the<br />
ends of the toothpicks will differ. However, the HCN model will have the longest<br />
distance since it is linear (180° bond angle).<br />
Make sure the students understand that lone pairs affect the actual bond angles. For<br />
example, H 2 S, a three atom molecule, has tetrahedral electron pair geometry but a bent<br />
shape with an angle of approximately 109.5°. It is not linear as students might expect<br />
since there are only two atoms bound to the central atom.<br />
HCN H 2 CO CCl 2 H 2<br />
O 3 H 2 S PH 3<br />
Analysis and Conclusions: Answer Key<br />
1. Which shape has the longest distance between the ends of the toothpicks? Which<br />
shape has the shortest distance between the ends of the toothpicks?<br />
The linear shape has the longest distance between the ends of the toothpicks. The<br />
tetrahedral shape has the shortest distance between the ends of the toothpicks.<br />
2. Group together molecules that have the same number of atoms coming off the<br />
central atom. Do these molecules have the same geometry? The same shape?<br />
2 atoms off the central atom: HCN, O 3 , H 2 S; 3 atoms: H 2 CO, PH 3 ; 4 atoms: CCl 2 H 2 . The<br />
molecules in a given group do not necessarily have the same electron pair geometry, or<br />
the same shape.<br />
3. What do the plain toothpicks represent? The colored toothpicks?