Universal role of correlation entropy in critical phenomena
Universal role of correlation entropy in critical phenomena
Universal role of correlation entropy in critical phenomena
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
J. Phys. A: Math. Theor. 41 (2008) 025002 S-J Gu et al<br />
S<br />
0.35<br />
0.3<br />
0.25<br />
0.2<br />
0.15<br />
0.1<br />
0.05<br />
0<br />
0<br />
10<br />
20<br />
r<br />
30<br />
40<br />
50 2.2<br />
2.22<br />
2.26<br />
2.24T<br />
2.28<br />
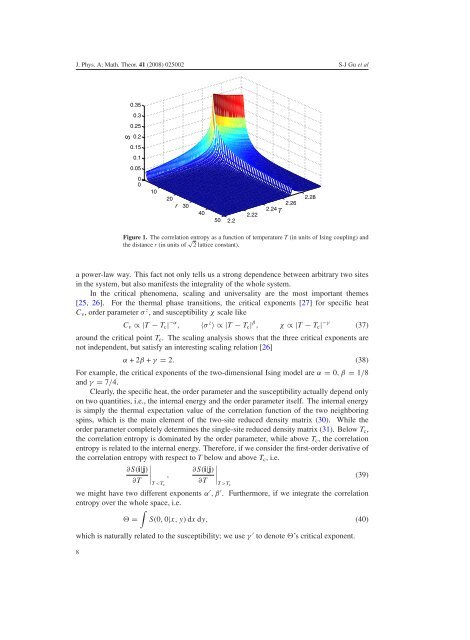
Figure 1. The <strong>correlation</strong> <strong>entropy</strong> as a function <strong>of</strong> temperature T (<strong>in</strong> units <strong>of</strong> Is<strong>in</strong>g coupl<strong>in</strong>g) and<br />
the distance r (<strong>in</strong> units <strong>of</strong> √ 2 lattice constant).<br />
a power-law way. This fact not only tells us a strong dependence between arbitrary two sites<br />
<strong>in</strong> the system, but also manifests the <strong>in</strong>tegrality <strong>of</strong> the whole system.<br />
In the <strong>critical</strong> <strong>phenomena</strong>, scal<strong>in</strong>g and universality are the most important themes<br />
[25, 26]. For the thermal phase transitions, the <strong>critical</strong> exponents [27] for specific heat<br />
C v , order parameter σ z , and susceptibility χ scale like<br />
C v ∝|T − T c | −α , 〈σ z 〉∝|T − T c | β , χ ∝|T − T c | −γ (37)<br />
around the <strong>critical</strong> po<strong>in</strong>t T c . The scal<strong>in</strong>g analysis shows that the three <strong>critical</strong> exponents are<br />
not <strong>in</strong>dependent, but satisfy an <strong>in</strong>terest<strong>in</strong>g scal<strong>in</strong>g relation [26]<br />
α +2β + γ = 2. (38)<br />
For example, the <strong>critical</strong> exponents <strong>of</strong> the two-dimensional Is<strong>in</strong>g model are α = 0,β = 1/8<br />
and γ = 7/4.<br />
Clearly, the specific heat, the order parameter and the susceptibility actually depend only<br />
on two quantities, i.e., the <strong>in</strong>ternal energy and the order parameter itself. The <strong>in</strong>ternal energy<br />
is simply the thermal expectation value <strong>of</strong> the <strong>correlation</strong> function <strong>of</strong> the two neighbor<strong>in</strong>g<br />
sp<strong>in</strong>s, which is the ma<strong>in</strong> element <strong>of</strong> the two-site reduced density matrix (30). While the<br />
order parameter completely determ<strong>in</strong>es the s<strong>in</strong>gle-site reduced density matrix (31). Below T c ,<br />
the <strong>correlation</strong> <strong>entropy</strong> is dom<strong>in</strong>ated by the order parameter, while above T c , the <strong>correlation</strong><br />
<strong>entropy</strong> is related to the <strong>in</strong>ternal energy. Therefore, if we consider the first-order derivative <strong>of</strong><br />
the <strong>correlation</strong> <strong>entropy</strong> with respect to T below and above T c , i.e.<br />
∂S(i|j)<br />
∂T<br />
∣ ,<br />
TTc<br />
we might have two different exponents α ′ ,β ′ . Furthermore, if we <strong>in</strong>tegrate the <strong>correlation</strong><br />
<strong>entropy</strong> over the whole space, i.e.<br />
∫<br />
= S(0, 0|x,y)dx dy, (40)<br />
which is naturally related to the susceptibility; we use γ ′ to denote ’s <strong>critical</strong> exponent.<br />
8