RNA Guide: Purifying RNA and mRNA - Promega
RNA Guide: Purifying RNA and mRNA - Promega
RNA Guide: Purifying RNA and mRNA - Promega
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Purifying</strong> <strong>RNA</strong> <strong>and</strong> m<strong>RNA</strong><br />
Purification Requirements<br />
The purity <strong>and</strong> integrity of <strong>RNA</strong> is critical to the success<br />
of any <strong>RNA</strong>-based analysis. Purification methods must<br />
protect <strong>RNA</strong> during <strong>and</strong> after purification.<br />
To successfully isolate intact <strong>RNA</strong> by any procedure,<br />
the methods must:<br />
1. Disrupt cells or tissue.<br />
2. Denature the nucleic acid:protein complexes.<br />
3. Inactivate endogenous ribonucleases.<br />
4. Purify the <strong>RNA</strong> from contaminating DNA <strong>and</strong><br />
protein.<br />
Do you need total <strong>RNA</strong> or poly(A)+ <strong>RNA</strong> for your<br />
application? For most applications, total <strong>RNA</strong> is<br />
sufficient, but in some<br />
cases the added<br />
sensitivity of poly(A)+<br />
<strong>RNA</strong> is beneficial. This<br />
is especially true if the<br />
<strong>RNA</strong> is intended for<br />
cDNA library<br />
construction or<br />
detection of very<br />
rare messages.<br />
RNasin ®<br />
Ribonuclease Inhibitor<br />
protects purified<br />
<strong>RNA</strong> in downstream<br />
applications.<br />
See page 3<br />
Look for these symbols to find the system<br />
right for your application.<br />
Animal Tissue<br />
Cultured Cells<br />
Blood<br />
<strong>Promega</strong> has a variety of solutions for your <strong>RNA</strong><br />
purification needs. All systems produce high-quality<br />
<strong>RNA</strong> ready for use from a variety of starting materials.<br />
The PureYield <strong>RNA</strong> Midiprep System uses a novel<br />
procedure for removal of genomic DNA from the <strong>RNA</strong><br />
prep without DNase treatment. The <strong>RNA</strong> is ideally suited<br />
for any application influenced by the presence of<br />
genomic DNA, such as quantitative, real-time RT-PCR.<br />
The SV, SV 96 <strong>and</strong> MagneSil ® Total <strong>RNA</strong> Isolation<br />
Systems include DNase for in-process genomic DNA<br />
removal. Both the SV 96 <strong>and</strong> MagneSil <strong>RNA</strong> Systems<br />
can be automated for high-throughput purification. The<br />
<strong>RNA</strong>gents ® System is a total scalable solutions-based<br />
system that uses organic extraction to produce high<br />
quality <strong>RNA</strong>.<br />
Plant Tissue<br />
5584MA<br />
Bacteria<br />
Yeast<br />
Please note, in the flow charts that follow, <strong>Promega</strong> systems<br />
are listed based on the protocols are provided in the Technical<br />
Literature sent with each system. Other applications with other<br />
source materials may exist.<br />
Please contact <strong>Promega</strong> Technical Services if you have<br />
questions: techserv@promega.com<br />
www.promega.com • techserv@promega.com 7
<strong>Purifying</strong> <strong>RNA</strong> <strong>and</strong> m<strong>RNA</strong><br />
Automated<br />
Animal<br />
Tissue<br />
Manual<br />
Unique product!<br />
Novel clearing agent<br />
removes all detectable<br />
genomic DNA.<br />
No DNase<br />
treatment needed!<br />
MagneSil ® Total <strong>RNA</strong><br />
mini-Isolation System<br />
SV 96 Total <strong>RNA</strong><br />
Isolation System<br />
PureYield <strong>RNA</strong><br />
Midiprep System<br />
Can process up to<br />
2mg of tissue lysate<br />
in 200µl of lysis<br />
buffer in the<br />
96-well format<br />
DNase included to<br />
greatly reduce<br />
gDNA carry-over.<br />
Scalable solution-based<br />
reagent that can<br />
process up to 1g of<br />
tissue in a single prep<br />
SV Total <strong>RNA</strong><br />
Isolation System<br />
<strong>RNA</strong>gents ® Total<br />
<strong>RNA</strong> Isolation system<br />
PolyATtract ® System<br />
1000<br />
5585MA<br />
Go from tissue to<br />
poly(A)+ <strong>RNA</strong><br />
directly.<br />
The SV Total <strong>RNA</strong> Isolation System has been cited for total<br />
<strong>RNA</strong> isolation from a wide variety of mammalian tissues<br />
including heart, lung, kidney, brain, eye, colon, small intestine,<br />
muscle, cochlea, stomach, placenta <strong>and</strong> thymus. Some more<br />
exotic tissues include:<br />
snake venom sacs & gl<strong>and</strong>s<br />
insect larvae<br />
whole water flea<br />
zebrafish gut<br />
carp eggs<br />
frog liver & gonads<br />
chicken heart, leg & brain<br />
marine polychaetes<br />
For more information, check the online citations<br />
database at: www.promega.com/citations or contact<br />
Technical Services at: techserv@promega.com<br />
8<br />
<strong>Promega</strong> <strong>RNA</strong> Analysis Notebook
<strong>Purifying</strong> <strong>RNA</strong> <strong>and</strong> m<strong>RNA</strong><br />
Cultured<br />
Cells<br />
Unique product!<br />
Novel clearing agent<br />
removes all detectable<br />
genomic DNA.<br />
No DNase<br />
treatment needed!<br />
Automated<br />
Manual<br />
MagneSil ® Total <strong>RNA</strong><br />
mini-Isolation System<br />
SV 96 Total <strong>RNA</strong><br />
Isolation System<br />
PureYield <strong>RNA</strong><br />
Midiprep System<br />
SV Total <strong>RNA</strong><br />
Isolation System<br />
Can process: ~10 5<br />
cells in 96-well<br />
format; s10 3 cells in<br />
384-well format<br />
Precipitation of <strong>RNA</strong> Samples<br />
DNase included to<br />
greatly reduce<br />
gDNA carry-over.<br />
Precipitation of <strong>RNA</strong> is commonly used for purification<br />
or concentration purposes. To ensure maximum<br />
recovery when precipitating small quantities (
<strong>Purifying</strong> <strong>RNA</strong> <strong>and</strong> m<strong>RNA</strong><br />
Automated<br />
Blood<br />
Manual<br />
<strong>RNA</strong> yield<br />
from blood<br />
varies with<br />
white cell count<br />
MagneSil ® Total <strong>RNA</strong><br />
mini-Isolation System<br />
PureYield <strong>RNA</strong><br />
Midiprep System<br />
SV Total <strong>RNA</strong><br />
Isolation System<br />
Unique product!<br />
Novel clearing agent<br />
removes all detectable<br />
genomic DNA.<br />
No DNase<br />
treatment needed!<br />
<strong>RNA</strong>gents ® Total<br />
<strong>RNA</strong> Isolation System<br />
Scalable solution-based<br />
reagent that can<br />
process up to 1gm of<br />
tissue in a single prep<br />
5587MA<br />
DNase included to<br />
greatly reduce<br />
gDNA carry-over.<br />
Can process 20µl<br />
whole blood in<br />
96-well format;<br />
5µl whole blood in<br />
384-well format<br />
Protocols require<br />
removal of red blood<br />
cells with SV Red<br />
Cell Lysis Solution<br />
prior to purification<br />
In addition to <strong>RNA</strong> isolation from mammalian<br />
blood, the SV Total <strong>RNA</strong> Isolation System has<br />
been used on related non-mammalian sources,<br />
including marine invertebrate hemolymph <strong>and</strong><br />
fish red blood cells.<br />
10<br />
<strong>Promega</strong> <strong>RNA</strong> Analysis Notebook
<strong>Purifying</strong> <strong>RNA</strong> <strong>and</strong> m<strong>RNA</strong><br />
Plant<br />
Tissue<br />
Manual<br />
PureYield <strong>RNA</strong><br />
Midiprep System<br />
SV Total <strong>RNA</strong><br />
Isolation System<br />
<strong>RNA</strong>gents ® Total<br />
<strong>RNA</strong> Isolation System<br />
5588MA<br />
Unique product!<br />
Novel clearing agent<br />
removes all detectable<br />
genomic DNA.<br />
No DNase<br />
treatment needed!<br />
DNase included to<br />
greatly reduce<br />
gDNA carry-over.<br />
Scalable solution-based<br />
reagent that can<br />
process up to 1gm of<br />
tissue in a single prep<br />
The SV Total <strong>RNA</strong> Isolation System has been cited for total <strong>RNA</strong> purification from tissues of the following:<br />
Arabidopsis thaliana<br />
Beta vulgaris<br />
Brassica napus<br />
Cichorium intybus<br />
Hordeum vulgare<br />
Lotus japonica<br />
Lycospersicon esculentum<br />
Manihot exculenta<br />
Medicago truncatula<br />
Nicotiana tabacum<br />
Pisum sativum<br />
Prunus cerasifera<br />
Trifolium repens<br />
Vigna sp.<br />
Vitis vinifera<br />
Tissues used for isolation include root, stem, leaves, seedlings <strong>and</strong> berries. For more information, check the online<br />
citations database at: www.promega.com/citations or contact Technical Services at: techserv@promega.com<br />
www.promega.com • techserv@promega.com 11
<strong>Purifying</strong> <strong>RNA</strong> <strong>and</strong> m<strong>RNA</strong><br />
Protocols require<br />
lysozyme <strong>and</strong>/or<br />
lysostaphin<br />
Bacteria<br />
Manual<br />
Protocols for<br />
Gram Positive &<br />
Gram Negative<br />
bacteria<br />
SV Total <strong>RNA</strong><br />
Isolation System<br />
DNase included to<br />
greatly reduce<br />
gDNA carry-over.<br />
PureYield <strong>RNA</strong><br />
Midiprep System<br />
Unique product!<br />
Novel clearing agent<br />
removes all detectable<br />
genomic DNA.<br />
No DNase<br />
treatment needed!<br />
5589MA<br />
The SV Total <strong>RNA</strong> Isolation System has been<br />
cited for total <strong>RNA</strong> purification from many<br />
bacteria. Some examples are:<br />
Alteromonas<br />
Bacillus<br />
Chlamydia<br />
Escherichia<br />
Klebsiella<br />
Lactobacillus<br />
Microcystis<br />
Mycobacterium<br />
Neisseria<br />
Orchrobactrum<br />
Photorhabdus<br />
Pseudomonas<br />
Rhizobium<br />
Rhodopseudomonas<br />
Salmonella<br />
Simkania<br />
Streptococcus<br />
Streptomyces<br />
Synechocystis<br />
Vibrio<br />
Yersinia<br />
For more information, check the online<br />
citations database at:<br />
www.promega.com/citations/<br />
or contact Technical Services at:<br />
techserv@promega.com<br />
Protocols require<br />
lyticase or<br />
zymolase<br />
Manual<br />
Yeast &<br />
Fungi<br />
The SV Total <strong>RNA</strong> Isolation System has been<br />
cited for total <strong>RNA</strong> purification from:<br />
Saccharomyces<br />
cerevisiae<br />
Blumeria grominis<br />
Bierk<strong>and</strong>era sp.<br />
Gigaspora margarita<br />
Trichophyton rubrum<br />
SV Total <strong>RNA</strong><br />
Isolation System<br />
PureYield <strong>RNA</strong><br />
Midiprep System<br />
5590MA<br />
12<br />
<strong>Promega</strong> <strong>RNA</strong> Analysis Notebook
<strong>Purifying</strong> <strong>RNA</strong> <strong>and</strong> m<strong>RNA</strong><br />
Scalable Total <strong>RNA</strong> Isolation<br />
<strong>Promega</strong>’s original solution-based system, the<br />
<strong>RNA</strong>gents ® Total <strong>RNA</strong> Isolation System is based on the<br />
classic method described by Chomczynski <strong>and</strong> Sacchi<br />
(1). We improved the method by developing a novel<br />
Denaturation Solution that reduces DNA carryover.<br />
This scalable system can be used with mammalian<br />
tissue, cultured cells <strong>and</strong> plant tissue <strong>and</strong> can be<br />
adapted to any sample size.<br />
10<br />
8<br />
<strong>RNA</strong>gents ® Total <strong>RNA</strong><br />
Isolation System<br />
Protocol available at:<br />
www.promega.com/tbs/tb087/<br />
tb087.html<br />
Cat.# Z5110<br />
Citations available at:<br />
www.promega.com/citations/<br />
1 2 3 4 5 6<br />
<strong>RNA</strong> Yield (mg)<br />
6<br />
4<br />
– 28S<br />
– 18S<br />
Formaldehyde gel electrophoresis of total <strong>RNA</strong>. Five micrograms of total <strong>RNA</strong><br />
isolated with <strong>RNA</strong>gents ® System from HeLa cells (lane 1), mouse intestine (lane 2), mouse<br />
spleen (lane 3), mouse lung (lane 4), mouse kidney (lane 5) <strong>and</strong> mouse liver (lane 6).<br />
0349TA11_5A<br />
2<br />
Add Sodium<br />
Acetate to<br />
prepared lysate.<br />
0<br />
0 0.2 0.4 0.6 0.8 1.0<br />
Tissue (g)<br />
Purification of total <strong>RNA</strong> from mouse liver with <strong>RNA</strong>gents ® Total <strong>RNA</strong><br />
Isolation System.<br />
1270MA11_5B<br />
Table 1. Yields <strong>and</strong> A 260 /A 280 Ratios of Total <strong>RNA</strong> purified<br />
with the <strong>RNA</strong>gents ® System.<br />
Source Yield of Total <strong>RNA</strong> A 260 /A 280<br />
HeLa Cells 1.6mg/10 8 cells 1.85<br />
Human WBC 1.3mg/10 8 cells 1.72<br />
Mouse Intestine 2.3mg/g tissue 1.75<br />
Mouse Spleen 8.3mg/g tissue 1.67<br />
Mouse Lung 1.9mg/g tissue 1.75<br />
Mouse Liver 6.6mg/g tissue 1.99<br />
Mouse Kidney 3.1mg/g tissue 1.70<br />
<strong>RNA</strong>gents ® Denaturing<br />
Solution is available separately<br />
<strong>and</strong> can be easily combined<br />
with your reagents providing<br />
an economical choice for<br />
<strong>RNA</strong> isolation.<br />
Add Phenol:<br />
Chloroform:<br />
Isoamyl Alcohol<br />
to tube, mix<br />
<strong>and</strong> chill.<br />
Transfer mixture<br />
<strong>and</strong> centrifuge.<br />
Transfer top aqueous<br />
phase to new tube.<br />
Add Isopropanol.<br />
Incubate at –20°C.<br />
Centrifuge, then<br />
discard supernatant.<br />
For RT-PCR go to "<strong>RNA</strong> Wash"<br />
The <strong>RNA</strong>gents ® Procedure.<br />
Add Denaturing<br />
Solution. Vortex.<br />
Add Isopropanol.<br />
Centrifuge, then<br />
discard supernatant.<br />
Add ice-cold<br />
75% ethanol.<br />
Centrifuge, then<br />
discard supernatant.<br />
Air-dry the <strong>RNA</strong><br />
pellet. Dissolve in<br />
water or TE buffer.<br />
Store.<br />
3513MA09_2B<br />
www.promega.com • techserv@promega.com 13
<strong>Purifying</strong> <strong>RNA</strong> <strong>and</strong> m<strong>RNA</strong><br />
Small-scale Single-prep<br />
Total <strong>RNA</strong> Isolation<br />
The single-prep SV Total <strong>RNA</strong> Isolation System offers<br />
speed <strong>and</strong> convenience. The column-based method<br />
can isolate total <strong>RNA</strong> from up to 60mg of mammalian<br />
tissue. Denaturation <strong>and</strong> inactivation of RNases are<br />
accomplished without the use of phenol. <strong>Promega</strong> was<br />
the first to offer a system that eliminates residual DNA<br />
by performing DNase digestion directly on the column<br />
membrane, greatly reducing DNA carryover.<br />
SV Total <strong>RNA</strong> Isolation System<br />
Protocol available at:<br />
www.promega.com/tbs/tm048/<br />
tm048.html<br />
Cat.# Z3100<br />
Citations available at:<br />
www.promega.com/citations/<br />
Max. Amount Avg.<br />
Sample Type Processed Yield<br />
Mouse Liver 30mg 131µg<br />
Mouse Kidney 20mg 44µg<br />
Mouse Spleen 15mg 79µg<br />
Mouse Brain 60mg 39µg<br />
Mouse Muscle 30mg 22µg<br />
Rat Pancreas 30mg 100µg<br />
Rat Heart 60mg 16µg<br />
Rat Lung 60mg 36µg<br />
Tomato Leaf 30mg 5µg<br />
E. coli 10 9 cells 36µg<br />
S. cerevisiae 4 × 10 7 cells 19µg<br />
Spin<br />
Spin for one minute.<br />
Wash with <strong>RNA</strong><br />
Wash Solution.<br />
DNase Treatment.<br />
Add DNase<br />
Stop Solution.<br />
Spin for one minute.<br />
Wash 2X with<br />
<strong>RNA</strong> Wash Solution.<br />
Elute <strong>RNA</strong> into<br />
Elution Tube.<br />
DNase<br />
treatment gives<br />
better results<br />
+DNase<br />
Homogenize sample (tissues,<br />
cultured cells or white blood cells)<br />
in <strong>RNA</strong> Lysis Buffer.<br />
Transfer 175µl to a fresh tube. Add<br />
<strong>RNA</strong> Dilution Buffer; mix <strong>and</strong> centrifuge.<br />
Transfer the cleared lysate to a fresh tube;<br />
add 95% Ethanol <strong>and</strong> mix.<br />
Transfer to Spin Column Assembly.<br />
Total time: 60-70 minutes.<br />
–DNase<br />
DNase<br />
included<br />
Vacuum<br />
Remove lysate<br />
by vacuum.<br />
Wash with <strong>RNA</strong><br />
Wash Solution.<br />
DNase Treatment.<br />
Add DNase Stop Solution.<br />
Wash 2X with <strong>RNA</strong><br />
Wash Solution.<br />
Remove Spin Basket <strong>and</strong> place<br />
in collection tube. Centrifuge.<br />
Place Spin Basket in Elution Tube.<br />
–1.2Kb<br />
–400bp<br />
1766MB04_1A<br />
Taken from Technical Manual #TM048<br />
3548TA09_1A<br />
RT-PCR amplification from total <strong>RNA</strong> purified from mouse liver lysate with or<br />
without DNase treatment. Amplification primers used for RT-PCR were designed to<br />
amplify both m<strong>RNA</strong> <strong>and</strong> DNA in the same reaction. Amplification products specific for the<br />
m<strong>RNA</strong> are 400bp, <strong>and</strong> amplification products for the gene are 1.2kb. Both the SV <strong>and</strong> SV<br />
96 Total <strong>RNA</strong> Isolation Systems include DNase for on-membrane DNase treatment to<br />
remove genomic DNA below detectable levels.<br />
14<br />
<strong>Promega</strong> <strong>RNA</strong> Analysis Notebook
<strong>Purifying</strong> <strong>RNA</strong> <strong>and</strong> m<strong>RNA</strong><br />
Mid-scale, Single-prep Total<br />
<strong>RNA</strong> Isolation<br />
As st<strong>and</strong>ard molecular biology applications become more<br />
sensitive, it is increasingly important for <strong>RNA</strong> isolations<br />
to be free of contaminating genomic DNA. The<br />
PureYield <strong>RNA</strong> Midiprep System uses a novel combination<br />
of reagents, membrane, <strong>and</strong> protocol to achieve<br />
pure <strong>RNA</strong> with undetectable genomic DNA contamination.<br />
The isolation is completed without the use of a<br />
DNase treatment, organic solvents, protease digestions,<br />
or alcohol precipitations. The eluted <strong>RNA</strong> is ready for<br />
sensitive downstream applications such as quantitative<br />
RT-PCR, RT-PCR, <strong>and</strong> microarray analysis.<br />
Amplification Curves<br />
No detectable<br />
gDNA by qPCR<br />
1. Add isopropanol to the<br />
cleared lysate.<br />
2. Mix <strong>and</strong> transfer to<br />
PureYield Binding Column.<br />
1. Prepare lysate.<br />
2. Add <strong>RNA</strong> Dilution Buffer. Mix.<br />
3. Add Clearing Agent. Mix <strong>and</strong> vortex.<br />
4. Incubate at 70°C for 5 minutes.<br />
5. Cool for 5 minutes.<br />
1. Transfer mixture to PureYield<br />
Clearing Column in collection tube.<br />
2. Centrifuge to clear the lysate.<br />
No DNase<br />
treatment<br />
required<br />
RFU<br />
-1.1E5 -1.0E5 -9.0E5 -8.0E5 -7.0E5 -6.0E5 -5.0E5 -4.0E5 -3.0E5 -2.0E5 -1.0E5 0<br />
DNA St<strong>and</strong>ards<br />
Competitor I<br />
No-Template Control<br />
PureYield<br />
<strong>RNA</strong> Midiprep<br />
5 10 15 20 25 30 35 40<br />
Cycle<br />
<strong>RNA</strong> purified with the PureYield <strong>RNA</strong> Midiprep System has no detectable<br />
genomic DNA contamination. <strong>RNA</strong> was purified from 1 × 10 8 HEK293T using the<br />
PureYield system <strong>and</strong> competitor “I’s” solution-based purification method. 100ng of<br />
purified total <strong>RNA</strong> was analyzed using the Plexor qPCR System to determine the<br />
quantity of gDNA using primers specific for the human thyroid peroxidase gene<br />
(TPOX). Human Genomic DNA (Cat.# G3051) in quantites of 10 4 , 10 3 , 10 2 <strong>and</strong><br />
10 1 copies was used as a st<strong>and</strong>ard.<br />
PureYield <strong>RNA</strong> Midiprep<br />
System<br />
Protocol available at:<br />
www.promega.com/tbs/tm279/tm<br />
279.html<br />
Cat. # Z3740 <strong>and</strong> Z3741<br />
5360TA<br />
Centrifuge (Spin)<br />
1. Centrifuge.<br />
2. Wash 2X.<br />
3. Centrifuge<br />
10 minutes<br />
to dry.<br />
Vacuum (Vac)<br />
1. Apply vacuum.<br />
2. Wash 2X.<br />
3. Dry 3 minutes.<br />
1. Transfer PureYield Binding<br />
Column to 50ml collection tube.<br />
2. Add Nuclease-Free Water<br />
<strong>and</strong> incubate 2 minutes.<br />
3. Centrifuge 3 minutes to elute<br />
pure <strong>RNA</strong>.<br />
Schematic representation of the PureYield Total <strong>RNA</strong> Isolation System.<br />
Average Yields of Total <strong>RNA</strong> Isolated from Tissues <strong>and</strong> Cells.<br />
Sample<br />
Sample Amount / Maxium Average Yield Average Average<br />
Type Sample Capacity per Prep (µg) 1 A 260 /A 230 A 260 /A 280<br />
Rat Tissues<br />
Liver 300mg 990.7 1.8 1.9<br />
Lung 300mg 193.9 2.0 2.1<br />
Kidney 200mg 329.1 2.3 2.1<br />
Spleen 150mg 430.9 2.3 2.1<br />
Brain 300mg 305.5 2.3 2.1<br />
Heart 300mg 255.3 2.2 2.1<br />
Muscle 300mg 115.1 2.1 2.1<br />
Bacteria<br />
E. coli 1 × 10 10 cells 872.7 2.5 2.1<br />
Plant Tissue<br />
Canola 300mg 87.8 1.5 2.1<br />
Cell Lines<br />
HEK 293T 5 × 10 7 cells 453.3 2.1 1.9<br />
HeLa 5 × 10 7 cells 329.2 1.8 2.0<br />
Blood 20ml (10ml/tube) ~10* * *<br />
1. The values represent means of results achieved at <strong>Promega</strong>. Yields will depend on the metabolic state of<br />
the sample, culture conditions, harvesting conditions <strong>and</strong> sample preparations. The average total <strong>RNA</strong> yield<br />
shown is from a 1ml elution.<br />
* Varies by white cell count. A white cell count of ~5 × 10 6 cells/ml yields ~10µg of total <strong>RNA</strong>.<br />
www.promega.com • techserv@promega.com 15<br />
5124MB
<strong>Purifying</strong> <strong>RNA</strong> <strong>and</strong> m<strong>RNA</strong><br />
96-Well Total <strong>RNA</strong> Isolation<br />
As your <strong>RNA</strong> isolation needs grow, <strong>Promega</strong> has the<br />
tools to increase your <strong>RNA</strong> isolation throughput. All the<br />
advantages of the single-prep SV Total <strong>RNA</strong> Isolation<br />
System are included in a 96-well format of the SV 96<br />
Total <strong>RNA</strong> Isolation System. The system uses a vacuum<br />
manifold to allow the isolation of <strong>RNA</strong> from an entire<br />
plate of 96 samples as efficiently as possible. Isolations<br />
can be scaled up for benchtop purification or for use<br />
with liquid-h<strong>and</strong>lers. Use the Vac-Man ® 96 Vacuum<br />
Manifold for benchtop <strong>and</strong> automated methods unless<br />
your robotic system requires a specific manifold.<br />
SV 96 Total <strong>RNA</strong> Isolation<br />
System<br />
Protocol available at:<br />
www.promega.com/tbs/tb294/<br />
tb294.html<br />
Cat.# Z3500, Z3505<br />
Automated protocols available.<br />
Apply sample lysate<br />
Binding Plate<br />
Bind <strong>RNA</strong>.<br />
Wash.<br />
DNase treat.<br />
DNase<br />
included<br />
Wash.<br />
Elute total <strong>RNA</strong>.<br />
3446CA06_1A<br />
Elution Plate<br />
Highly pure total <strong>RNA</strong>.<br />
2651MB03_1A<br />
16<br />
Comparison of SV <strong>and</strong> SV 96 Total <strong>RNA</strong> Isolation<br />
Yields<br />
Total Yield (µg)<br />
0.8<br />
0.7<br />
0.6<br />
0.5<br />
0.4<br />
0.3<br />
0.2<br />
0.1<br />
SV 96<br />
If it works in SV,<br />
it’ll work in SV 96!<br />
0<br />
1 × 10 5 Cells<br />
Vacuum<br />
Spin<br />
St<strong>and</strong>ard SV Total<br />
<strong>RNA</strong> preps<br />
3806MA08_2A<br />
β-actin –<br />
Go directly from<br />
eluted <strong>RNA</strong> to<br />
RT-PCR!<br />
RT-PCR from a Decreasing Number of SH-SY5Y<br />
Human Neuroblastoma Cells<br />
SH-SY5Y/SV96<br />
50k<br />
25k<br />
12.5k<br />
6,250<br />
3,125<br />
1,562<br />
781<br />
391<br />
195<br />
98<br />
49<br />
24<br />
<strong>RNA</strong> was isolated from individual wells containing the indicated number of<br />
cells using the SV 96 Total <strong>RNA</strong> Isolation System. Four microliters of each eluate<br />
were amplified with the Access RT-PCR System (Cat.# A1250) <strong>and</strong> β-Actin Primer Pair<br />
(Cat.# G5740).<br />
<strong>Promega</strong> <strong>RNA</strong> Analysis Notebook<br />
3344TA03_1A
<strong>Purifying</strong> <strong>RNA</strong> <strong>and</strong> m<strong>RNA</strong><br />
µg<br />
0.7<br />
0.6<br />
0.5<br />
0.4<br />
0.3<br />
0.2<br />
0.1<br />
0<br />
<strong>RNA</strong> isolated with the<br />
SV 96 Total <strong>RNA</strong> System can<br />
go directly into quantitative<br />
RT-PCR reactions.<br />
HeLa NIH 3T3 CHO<br />
SV96<br />
3467MA06_1A<br />
∆Rn<br />
5.0<br />
4.0<br />
3.0<br />
2.0<br />
1.0<br />
0<br />
0 5 10 15 20 25 30 35 40<br />
Cycle<br />
Number of HeLa Cells<br />
50,000 12,500<br />
25,000 6,250<br />
3,125<br />
1,563<br />
4068TA03_3B<br />
Ratio A 260 /A 280<br />
2.5<br />
2.0<br />
1.5<br />
1.0<br />
0.5<br />
0<br />
HeLa NIH 3T3 CHO<br />
SV96<br />
Ribosomal <strong>RNA</strong> Sizes<br />
Species r<strong>RNA</strong> Size (kb)<br />
Human 18S 1.9<br />
28S 5.0<br />
Mouse 18S 1.9<br />
28S 4.7<br />
Drosophila 18S 2.2<br />
28S 2.8<br />
Tobacco 16S 1.5<br />
18S 1.9<br />
23S 2.9<br />
25S 3.7<br />
Yeast 18S 2.0<br />
26S 3.8<br />
E. coli 16S 1.5<br />
23S 2.9<br />
3468MA06_1A<br />
Real-time quantitative RT-PCR analysis of lamin A/C message. Total <strong>RNA</strong><br />
isolated from indicated number of HeLa cells <strong>and</strong> eluted in 100µl of Nuclease-Free Water.<br />
Twenty microliters were used in a 100µl RT reaction <strong>and</strong> 5µl transferred to a 50µl<br />
quantitative, real-time PCR reaction. Further details in Brisco, P. <strong>and</strong> Hooper, K. (2003)<br />
Quantitative, real-time RT-PCR expression using the SV 96 Total <strong>RNA</strong> Isolation System.<br />
<strong>Promega</strong> Notes 84, 23–26.<br />
Storage of purified <strong>RNA</strong><br />
<strong>RNA</strong> should be stored in a dedicated container at<br />
–70°C or below. To avoid multiple freeze-thaw cycles<br />
<strong>and</strong> contamination, dispense the purified <strong>RNA</strong> into<br />
convenient volumes. For long-term storage or storage<br />
of critical samples, precipitate <strong>RNA</strong> aliquots by adding<br />
1/10th volume of 3M sodium acetate <strong>and</strong> 2 volumes<br />
of ethanol. Store the precipitated <strong>RNA</strong> under ethanol<br />
at –70°C or below.<br />
SV 96 Total <strong>RNA</strong><br />
works on plant <strong>and</strong><br />
mammalian tissues.<br />
Tomato Corn Tobacco Barley Wheat Alfalfa Arabid. Soy Rice<br />
M L S L S L S L S L S L S M L L L<br />
3509TA07_1B<br />
Isolation of total <strong>RNA</strong> from either leaf (L) or stem (S) tissues of various plant<br />
species. B<strong>and</strong>s indicate 28S, 18S <strong>and</strong> chloroplast r<strong>RNA</strong>s. Twenty microliters of each<br />
elution were loaded on a 1% gel stained with ethidium bromide. Protocol detailed in<br />
Grunst, T. (2001) High-throughput isolation with the SV 96 Total <strong>RNA</strong> Isolation System.<br />
<strong>Promega</strong> Notes 79, 29–32.<br />
www.promega.com • techserv@promega.com<br />
17
<strong>Purifying</strong> <strong>RNA</strong> <strong>and</strong> m<strong>RNA</strong><br />
96-Well or 384-Well Total<br />
<strong>RNA</strong> Isolation<br />
The MagneSil ® Total <strong>RNA</strong> mini-Isolation System<br />
provides a high-throughput 96- or 384-well format for<br />
fast, simple preparation of intact, purified total <strong>RNA</strong><br />
from small amounts of cultured cells, tissue, or fresh<br />
whole blood samples. The system is designed to<br />
perform four plates worth of purification in a 96- or<br />
384-well plate format. Total <strong>RNA</strong> isolation is achieved<br />
without the need for vacuum filtration, centrifugation or<br />
precipitation. The procedure is specifically geared for<br />
automated liquid h<strong>and</strong>lers.<br />
Add sample lysate to<br />
MagneSil ® <strong>RNA</strong> PMPs. Mix.<br />
Capture PMPs.<br />
Discard supernatant.<br />
Wash PMPs.<br />
Incubate PMPs with<br />
DNase 10 minutes.<br />
Add DNase Stop Solution.<br />
Elute <strong>RNA</strong> from a<br />
96-well purification<br />
in as little as 15µl<br />
Capture PMPs.<br />
Discard supernatant.<br />
Wash PMPs.<br />
Concentration (ng/µl)<br />
30<br />
20<br />
10<br />
0<br />
10 15 20 25 30 35 40 45 50<br />
Elute <strong>RNA</strong>.<br />
Schematic diagram of the MagneSil ® Total <strong>RNA</strong> mini-Isolation System<br />
protocol.<br />
4316MB11_3A<br />
Elution Volume (µl)<br />
Yield (µg)<br />
0.6<br />
0.4<br />
0.2<br />
0.0<br />
10 15 20 25 30 35 40 45 50<br />
Elution Volume (µl)<br />
Flexibility in elution volume to control sample concentration. Total <strong>RNA</strong> isolated<br />
from 2 × 10 4 HeLa cells/well using MagneSil Total <strong>RNA</strong> mini-Isolation System in a 96-well<br />
format. Titration of elution volume shown. Total <strong>RNA</strong> concentration <strong>and</strong> yield calculated by<br />
measurement of isolated total <strong>RNA</strong> with Molecular Probes RiboGreen ® assay.<br />
4377MA11_3A<br />
Maximum starting material for MagneSil ® Total <strong>RNA</strong><br />
mini-isolation System protocols:<br />
Sample 96-well 384-well<br />
Tissue Lysate<br />
Not<br />
(in 100µl lysis buffer) 2mg recommended<br />
Cultured Cells 10 5 10 3<br />
Whole Blood 20µl 5µl<br />
The mobile stationary phase<br />
of magnetic particles allows<br />
elution in smaller volumes than<br />
membrane-based <strong>RNA</strong><br />
purification systems<br />
18<br />
<strong>Promega</strong> <strong>RNA</strong> Analysis Notebook
<strong>Purifying</strong> <strong>RNA</strong> <strong>and</strong> m<strong>RNA</strong><br />
Ready for quantitative,<br />
real-time RT-PCR<br />
A.<br />
∆Rn<br />
Ct<br />
10 2<br />
10 1<br />
10 0<br />
10 –1<br />
10 –2<br />
40<br />
30<br />
20<br />
10 3<br />
10 4 10 2<br />
10 5 10<br />
10 –3 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 38 40<br />
Cycle<br />
GAPDH<br />
negative control<br />
Automated Methods for:<br />
• Beckman Coulter Biomek ® 2000<br />
• Beckman Coulter Biomek ® FX<br />
• Tecan Te-MO<br />
• Xiril X100<br />
Only the Biomek ® FX <strong>and</strong> Te-MO can apply the 384-well<br />
method. Some instruments may need extra hardware to<br />
automate this system For more information go to:<br />
www.promega.com/automethods/<br />
If you don’t have one of the instruments listed, you may<br />
be able to adapt the system to your instrument. Our<br />
reagent manuals include information such as how much<br />
of each reagent are required at each step to assist with<br />
the adaptation.<br />
10<br />
B.<br />
∆Rn<br />
10 0<br />
10 –1<br />
0<br />
1<br />
10<br />
100<br />
1,000<br />
Cell Number<br />
10,000<br />
100,000<br />
1E+06<br />
10 1 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 38 40<br />
10 5 10 4 10 3 10 2<br />
MagneSil ® Total <strong>RNA</strong><br />
mini-isolation System Protocol<br />
available at:<br />
www.promega.com/tbs/tb328/<br />
tb328.html<br />
Cat.# Z3351<br />
10 –2<br />
10<br />
Automation <strong>and</strong> magnetic st<strong>and</strong>s<br />
required for use.<br />
10 –3<br />
Cycle<br />
40<br />
c-myc<br />
30<br />
Ct<br />
20<br />
10<br />
0<br />
1<br />
10<br />
100<br />
1,000<br />
Cell Number<br />
10,000<br />
100,000<br />
1E+06<br />
4378TA11_3A<br />
Real-time RT-PCR analysis of purified <strong>RNA</strong>. 20µl aliquots of total <strong>RNA</strong> isolated<br />
from a 10X dilution series of HeLa cells seeded in a 96-well plate were used as template<br />
for reverse transcription (100µl reaction). Panel A: 5µl aliquots (n=3) of the RT reaction<br />
were used for PCR of GAPDH target. Panel B: 5µl aliquots (n=1) of the RT reaction were<br />
used for PCR of a c-Myc target. GAPDH signal (abundant m<strong>RNA</strong>) <strong>and</strong> c-Myc (rare m<strong>RNA</strong>)<br />
could be detected in as few as 10 HeLa cells.<br />
www.promega.com • techserv@promega.com 19
<strong>Purifying</strong> <strong>RNA</strong> <strong>and</strong> m<strong>RNA</strong><br />
Poly(A)+ m<strong>RNA</strong> Isolation<br />
Many applications are improved by isolating poly(A)+<br />
<strong>RNA</strong> either directly from the eukaryotic source material<br />
or from total <strong>RNA</strong> previously isolated from the source<br />
material. Some methods use oligo(dT) immobilized on a<br />
support such as cellulose or sepharose. These methods<br />
are prone to ribosomal <strong>RNA</strong> carryover. <strong>Promega</strong>’s<br />
PolyATtract ® Systems use solution hybridization of<br />
Poly(A)+ <strong>RNA</strong> to a biotinylated oligo(dT) followed by<br />
capture with a streptavidin-coated paramagnetic<br />
particle. The rapid hybridization <strong>and</strong> magnetic<br />
separation greatly improve the speed, efficiency <strong>and</strong><br />
quality of m<strong>RNA</strong> isolations.<br />
The PolyATtract ® m<strong>RNA</strong> Isolation Systems can be used<br />
if you are starting with total <strong>RNA</strong>. The kits are designed<br />
to purify Poly(A)+ <strong>RNA</strong> from 1–5mg or 0.1–1mg of total<br />
<strong>RNA</strong>. Reactions are scalable to meet your needs.<br />
To save time, isolate poly(A)+ <strong>RNA</strong> directly from starting<br />
material using the PolyATtract ® System 1000. The<br />
system uses a guanidine solution to disrupt the tissue<br />
<strong>and</strong> inhibit RNases, followed by the PolyATtract ®<br />
methodology for the isolation of Poly(A)+ <strong>RNA</strong>.<br />
<strong>Promega</strong> has published applications for mammalian<br />
<strong>and</strong> plant tissues.<br />
From Total <strong>RNA</strong>...<br />
PolyATtract ® m<strong>RNA</strong> Isolation System<br />
Protocols available at:<br />
www.promega.com/tbs/tm021/tm021.html<br />
Cat.# Z5200, Z5210, Z5300, Z5310<br />
Citations available at:<br />
www.promega.com/citations/<br />
total <strong>RNA</strong> containing<br />
m<strong>RNA</strong> fraction<br />
5′ m7G AAAAAAA<br />
3′<br />
Hybridize with<br />
biotin-oligo(dT).<br />
5′ m7G AAAAAAA 3′<br />
3′ TTTTTTT-B<br />
5′<br />
Add streptavidin PMPs.<br />
PMP<br />
5′ m7G AAAAAAA<br />
3′<br />
TTTTTTT-B<br />
Magnetize.<br />
Wash <strong>and</strong> elute.<br />
5′ B-TTTTTTT<br />
3′<br />
PMP<br />
5′ m7G AAAAAAA<br />
3′<br />
TTTTTTT-B<br />
PMP<br />
MAGNET<br />
Direct Isolation...<br />
PolyATtract ® System 1000<br />
Protocols available at:<br />
www.promega.com/tbs/tm228/tm228.html<br />
Cat.# Z5420, Z5400<br />
See citations at:<br />
www.promega.com/citations/<br />
5′ m7G AAAAAAA<br />
3′<br />
(aqueous)<br />
Solution-based<br />
hybridization to oligo(dT)<br />
is more efficient than<br />
hybridization to an<br />
immobilized oligo(dT)<br />
TTTTTTT-B<br />
(solid)<br />
PMP<br />
MAGNET<br />
µg total <strong>RNA</strong> µg m<strong>RNA</strong><br />
12 6 3 1.5 2 1 0.5 M<br />
0369MA03_1A<br />
20<br />
Prokaryotic m<strong>RNA</strong> enrichment<br />
Bacterial m<strong>RNA</strong> does not have the poly(A) tail<br />
characteristic of eukaryotic m<strong>RNA</strong>. Other tactics<br />
must be employed if you wish to get at the bacterial<br />
m<strong>RNA</strong>. Su <strong>and</strong> Sordillo (1998) used a probe directed<br />
to bacterial r<strong>RNA</strong> to remove the r<strong>RNA</strong> from a total<br />
bacterial <strong>RNA</strong> prep to enrich the sample for the<br />
bacterial m<strong>RNA</strong>.<br />
Su, C. <strong>and</strong> Sordillo, L.M. (1998) A simple method to<br />
enrich m<strong>RNA</strong> from total prokaryotic <strong>RNA</strong>. Mol.<br />
Biotechnol. 10, 83–85.<br />
Northern blot analysis<br />
comparing total <strong>RNA</strong> <strong>and</strong><br />
poly(A)+ <strong>RNA</strong> isolated<br />
from mouse liver. The<br />
Northern blot was probed for<br />
low abundance α-1-proteinase<br />
inhibitor message. Poly(A)+<br />
<strong>RNA</strong> was isolated directly from<br />
the tissue with the<br />
PolyATtract ® System 1000.<br />
<strong>Promega</strong> <strong>RNA</strong> Analysis Notebook<br />
0417TA06_2A
<strong>Purifying</strong> <strong>RNA</strong> <strong>and</strong> m<strong>RNA</strong><br />
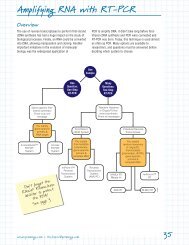
Downstream Applications<br />
Expression Profiling<br />
See chapter 5, Analyzing <strong>RNA</strong> with Microarrays.<br />
See chapter 3, Quantifying <strong>RNA</strong> with qRT-PCR.<br />
RT-PCR<br />
See chapter 4, Amplifying <strong>RNA</strong> with RT-PCR.<br />
Primer Extension Analysis<br />
Primer extension is used to quantitate <strong>and</strong> determine<br />
the location of the 5′-end of specific <strong>RNA</strong>s. With this<br />
technique, a radiolabeled DNA primer complementary to<br />
the <strong>RNA</strong> being studied is hybridized to the target <strong>and</strong><br />
extended using the enzymatic properties of reverse<br />
transcriptase. The DNA primer is designed to anneal to<br />
sequences near the 5′-end of the <strong>RNA</strong> target, <strong>and</strong> the<br />
extension reaction terminates when the reverse<br />
transcriptase reaches the extreme 5′-end of the <strong>RNA</strong>.<br />
The length of the cDNA product accurately defines the<br />
distance between the 5′-end of the radiolabeled primer<br />
<strong>and</strong> the 5′-end of the <strong>RNA</strong> (transcriptional start site).<br />
The quantity of cDNA produced is proportional to the<br />
amount of target <strong>RNA</strong>.<br />
Primer Extension System<br />
Protocol available at:<br />
www.promega.com/tbs/<br />
tb113/tb113.html<br />
Cat.# E3030<br />
Need information on other<br />
applications?<br />
Contact <strong>Promega</strong> Technical Services<br />
techserv@promega.com<br />
Nuclease Protection<br />
The nuclease protection assay is a sensitive technique<br />
used for the detection <strong>and</strong> quantitation of target <strong>RNA</strong><br />
sequences <strong>and</strong> related <strong>RNA</strong>s (6). A radiolabeled DNA or<br />
<strong>RNA</strong> probe is allowed to hybridize to target <strong>RNA</strong> in<br />
solution. After hybridization, the remaining singlestr<strong>and</strong>ed<br />
probe is removed from the reaction by<br />
incubation with either a ribonuclease, if the probe was<br />
<strong>RNA</strong> (RNase protection), or S1 nuclease, if the probe<br />
was DNA (S1 assay). Reaction products are resolved by<br />
polyacrylamide gel electrophoresis to quantitate the<br />
amount of protected probe. The technique is extremely<br />
sensitive, doesn’t require transfer of <strong>RNA</strong> to a solid<br />
support, <strong>and</strong> multiple <strong>RNA</strong> species can be probed in a<br />
single reaction. See the chapter called “Making <strong>RNA</strong> in<br />
vitro” for information about making <strong>RNA</strong> probes.<br />
<strong>Promega</strong> also offers<br />
RNase ONE<br />
Ribonuclease, a<br />
highly efficient<br />
ribonuclease that<br />
cleaves after each<br />
base in <strong>RNA</strong> so you<br />
won’t have to use<br />
mixtures like RNase A<br />
<strong>and</strong> RNase T1.<br />
RNase ONE Ribonuclease<br />
Cat.# M4261<br />
Citations available at:<br />
www.promega.com/citations/<br />
Northern Blotting<br />
Northern blotting is the classic technique used to<br />
examine the expression profile of m<strong>RNA</strong> following a<br />
specific treatment. Northern analysis has given way to<br />
techniques like RT-PCR <strong>and</strong> microarray analysis. For a<br />
complete method for Northern blotting, see the<br />
Expression Analysis chapter of the Protocols <strong>and</strong><br />
Applications <strong>Guide</strong>, available online at:<br />
www.promega.com/paguide.<br />
Protocols & Applications<br />
<strong>Guide</strong> at:<br />
www.promega.com/paguide<br />
www.promega.com • techserv@promega.com 21
<strong>Purifying</strong> <strong>RNA</strong> <strong>and</strong> m<strong>RNA</strong><br />
Total <strong>RNA</strong> Purification Products Size Cat.#<br />
SV Total <strong>RNA</strong> Isolation System (d) * 10 preps Z3101<br />
50 preps Z3100<br />
PureYield <strong>RNA</strong> Midiprep System (c,e) 10 preps Z3740<br />
50 preps Z3741<br />
SV 96 Total <strong>RNA</strong> Isolation System* 1 × 96 Z3500<br />
5 × 96 Z3505<br />
<strong>RNA</strong>gents ® Total <strong>RNA</strong> Isolation System* Scalable Z5110<br />
MagneSil ® Total <strong>RNA</strong> mini-Isolation System (f) 4 plate Z3351<br />
Items Available Separately<br />
SV <strong>RNA</strong> Lysis Buffer* 50ml Z3051<br />
SV <strong>RNA</strong> Red Cell Lysis Buffer* 200ml Z3141<br />
Used to lyse red blood cells prior to isolation of <strong>RNA</strong> from the nucleated white blood cells.<br />
Vac-Man ® Laboratory Vacuum Manifold 1 each A7231<br />
For use with the SV Total <strong>RNA</strong> System in a vacuum format.<br />
Twenty samples can be processed at once.<br />
Vacuum Adapters 20 each A1331<br />
Required for use with the Vac-Man ® Laboratory Vacuum Manifold <strong>and</strong> the<br />
SV Total <strong>RNA</strong> Isolation System in a vacuum format.<br />
Vac-Man ® 96 Vacuum Manifold 1 each A2291<br />
Required for use of the SV 96 Total <strong>RNA</strong> Isolation System.<br />
<strong>RNA</strong>gents ® Denaturing Solution 120ml Z5651<br />
m<strong>RNA</strong> Purification Products Size Cat.#<br />
PolyATtract ® m<strong>RNA</strong> Isolation System I* 3 isolations Z5200<br />
1–5mg total <strong>RNA</strong>; Magnetic St<strong>and</strong> included<br />
PolyATtract ® m<strong>RNA</strong> Isolation System II* 3 isolations Z5210<br />
Refill system for Z5200; no magnetic st<strong>and</strong><br />
PolyATtract ® m<strong>RNA</strong> Isolation System III* 15 isolations Z5300<br />
0.1–1mg total <strong>RNA</strong>; Magnetic St<strong>and</strong> included<br />
PolyATtract ® m<strong>RNA</strong> Isolation System IV* 15 isolations Z5310<br />
Refill system for Z5300; no magnetic st<strong>and</strong><br />
PolyATtract ® System 1000 with Magnetic St<strong>and</strong>* 3 isolations Z5420<br />
PolyATtract ® System 1000 without Magnetic St<strong>and</strong>* 3 isolations Z5400<br />
*For Laboratory Use.<br />
References<br />
1. Chomczynski, P. <strong>and</strong> Sacchi, N. (1987) Single-step method of <strong>RNA</strong> isolation by<br />
acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem.<br />
162, 156–159.<br />
2. Marcus, L. et al. (1996) PolyATtract ® Systems for m<strong>RNA</strong> purification. <strong>Promega</strong><br />
Notes 60, 14–16.<br />
3. Murillo, I. et al. (1995) Isoaltion of total <strong>RNA</strong> <strong>and</strong> m<strong>RNA</strong> from plant tissues.<br />
<strong>Promega</strong> Notes, 2–5.<br />
4. Rhodes, R. <strong>and</strong> Kephart, D. (1996) Automated Robotic Isolation of Poly(A)+<br />
m<strong>RNA</strong> Using PolyATtract ® m<strong>RNA</strong> Isolation Reagent. <strong>Promega</strong> Notes 75, 10–13.<br />
5. Barlow, J.J. et al. (1963) A simple method for quantitative isolation of<br />
undegraded high molecular weight ribonucleic acid. Biochem. Biophys. Res.<br />
Comm. 13, 61–66.<br />
6. Ausubel, F.M. et al. (2000) Current Protocols in Molecular Biology, Vol. 2, John<br />
Wiley & Sons, New York, 4.6–4.7.<br />
22<br />
<strong>Promega</strong> <strong>RNA</strong> Analysis Notebook