Annual Report 2002 - Roche
Annual Report 2002 - Roche
Annual Report 2002 - Roche
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
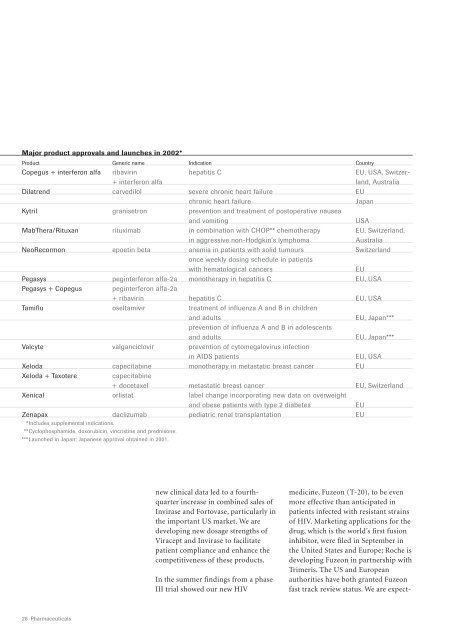
Major product approvals and launches in <strong>2002</strong>*<br />
Product Generic name Indication Country<br />
Copegus + interferon alfa ribavirin hepatitis C EU, USA, Switzer-<br />
+ interferon alfa land, Australia<br />
Dilatrend carvedilol severe chronic heart failure EU<br />
chronic heart failure<br />
Japan<br />
Kytril granisetron prevention and treatment of postoperative nausea<br />
and vomiting<br />
USA<br />
MabThera/Rituxan rituximab in combination with CHOP** chemotherapy EU, Switzerland,<br />
in aggressive non-Hodgkin’s lymphoma<br />
Australia<br />
NeoRecormon epoetin beta anemia in patients with solid tumours Switzerland<br />
once weekly dosing schedule in patients<br />
with hematological cancers<br />
EU<br />
Pegasys peginterferon alfa-2a monotherapy in hepatitis C EU, USA<br />
Pegasys + Copegus peginterferon alfa-2a<br />
+ ribavirin hepatitis C EU, USA<br />
Tamiflu oseltamivir treatment of influenza A and B in children<br />
and adults<br />
EU, Japan***<br />
prevention of influenza A and B in adolescents<br />
and adults<br />
EU, Japan***<br />
Valcyte valganciclovir prevention of cytomegalovirus infection<br />
in AIDS patients<br />
EU, USA<br />
Xeloda capecitabine monotherapy in metastatic breast cancer EU<br />
Xeloda + Taxotere<br />
capecitabine<br />
+ docetaxel metastatic breast cancer EU, Switzerland<br />
Xenical orlistat label change incorporating new data on overweight<br />
and obese patients with type 2 diabetes<br />
EU<br />
Zenapax daclizumab pediatric renal transplantation EU<br />
***Includes supplemental indications.<br />
***Cyclophosphamide, doxorubicin, vincristine and prednisone.<br />
***Launched in Japan; Japanese approval obtained in 2001.<br />
new clinical data led to a fourthquarter<br />
increase in combined sales of<br />
Invirase and Fortovase, particularly in<br />
the important US market. We are<br />
developing new dosage strengths of<br />
Viracept and Invirase to facilitate<br />
patient compliance and enhance the<br />
competitiveness of these products.<br />
In the summer findings from a phase<br />
III trial showed our new HIV<br />
medicine, Fuzeon (T-20), to be even<br />
more effective than anticipated in<br />
patients infected with resistant strains<br />
of HIV. Marketing applications for the<br />
drug, which is the world’s first fusion<br />
inhibitor, were filed in September in<br />
the United States and Europe; <strong>Roche</strong> is<br />
developing Fuzeon in partnership with<br />
Trimeris. The US and European<br />
authorities have both granted Fuzeon<br />
fast track review status. We are expect-<br />
26 Pharmaceuticals