Annual Report 2002 - Roche
Annual Report 2002 - Roche
Annual Report 2002 - Roche
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
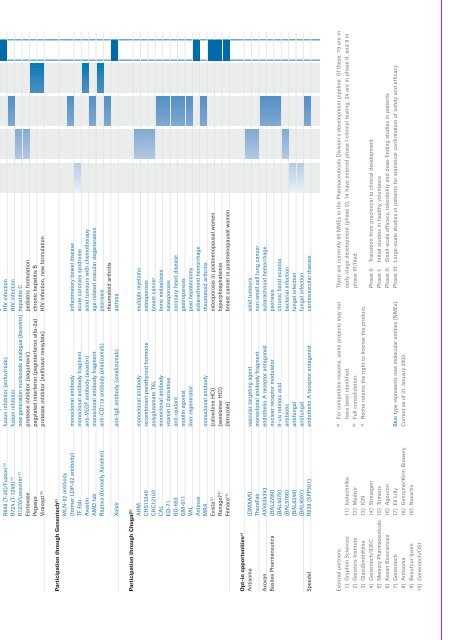
R698 (T-20)/Fuzeon 15) fusion inhibitor (enfuvirtide) HIV infection<br />
R724 (T-1249) 15) fusion inhibitor HIV infection<br />
R1270/Levovirin 13) new generation nucleoside analogue (levovirin) hepatitis C<br />
Fortovase protease inhibitor (saquinavir) pediatric formulation<br />
Pegasys pegylated interferon (peginterferon alfa-2a) chronic hepatitis B<br />
Viracept 16) protease inhibitor (nelfinavir mesylate) HIV infection, new formulation<br />
Participation through Genentech b)<br />
MLN-02 antibody<br />
(former LDP-02 antibody) monoclonal antibody inflammatory bowel disease<br />
TF Fab monoclonal antibody fragment acute coronary syndrome<br />
Avastin anti-VEGF antibody (avastin) solid tumours with chemotherapy<br />
AMD fab monoclonal antibody fragment age-related macular degeneration<br />
Raptiva (formally Xanelim) anti-CD11a antibody (efalizumab) psoriasis<br />
rheumatoid arthritis<br />
Xolair anti-IgE antibody (omalizumab) asthma<br />
Participation through Chugai b)<br />
AHM monoclonal antibody multiple myeloma<br />
CHS13340 recombinant parathyroid hormone osteoporosis<br />
CHC12103 polyglutamate TXL breast cancer<br />
CAL monoclonal antibody bone metastases<br />
ED-71 vitamin D derivative osteoporosis<br />
BO-653 anti-oxidant coronary heart disease<br />
GM-611 motilin agonist gastroparesis<br />
VAL liver regenerator post hepatectomy<br />
Antevas subarachnoid hemorrhage<br />
MRA monoclonal antibody rheumatoid arthritis<br />
Evista 17) (raloxifene HCl) osteoporosis in postmenopausal women<br />
Renagel 18) (sevelamer HCl) hyperphosphatemia<br />
Femara 19) (letrozole) breast cancer in postmenopausal women<br />
Opt-in opportunities c)<br />
Antisoma (DMXAA) vascular targeting agent solid tumours<br />
TheraFab monoclonal antibody fragment non-small cell lung cancer<br />
Axovan AXV034343 endothelin A receptor antagonist subarachnoid hemorrhage<br />
Basilea Pharmaceutica (BAL2299) nuclear receptor modulator psoriasis<br />
(BAL4079) 9-cis retinoic acid chronic hand eczema<br />
(BAL5788) antibiotic bacterial infection<br />
(BAL8349) antifungal fungal infection<br />
(BAL8557) antifungal fungal infection<br />
Speedel R639 (SPP301) endothelin A receptor antagonist cardiovascular disease<br />
External partners:<br />
1) Gryphon Sciences<br />
2) Genetics Institute<br />
3) GlaxoSmithKline<br />
4) Genentech/IDEC<br />
5) Memory Pharmaceuticals<br />
6) Kosan Biosciences<br />
7) Genentech<br />
8) Antisoma<br />
9) Beaufour Ipsen<br />
10) Genentech/OSI<br />
11) Isotechnika<br />
12) Medivir<br />
13) ICN<br />
14) Stressgen<br />
15) Trimeris<br />
16) Agouron<br />
17) Eli Lilly<br />
18) Genzyme/Kirin Brewery<br />
19) Novartis<br />
a)<br />
For competitive reasons, some projects may not<br />
have been identified.<br />
b)<br />
Full consolidation.<br />
c)<br />
<strong>Roche</strong> retains the right to license the product.<br />
Blue type represents new molecular entities (NMEs).<br />
Current as of 31 January 2003.<br />
There are currently 65 NMEs in the Pharmaceuticals Division’s development pipeline. Of these, 19 are in<br />
early-stage development (phase 0), 14 have entered phase I clinical testing, 24 are in phase II, and 8 in<br />
phase III/filed.<br />
Phase 0: Transition from preclinical to clinical development<br />
Phase I: Initial studies in healthy volunteers<br />
Phase II: Small-scale efficacy, tolerability and dose-finding studies in patients<br />
Phase III: Large-scale studies in patients for statistical confirmation of safety and efficacy