The operations of Eisai Co., Ltd. focus on - Eisai GmbH
The operations of Eisai Co., Ltd. focus on - Eisai GmbH
The operations of Eisai Co., Ltd. focus on - Eisai GmbH
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
To Our Shareholders<br />
<str<strong>on</strong>g>Eisai</str<strong>on</strong>g>’s corporate philosophy <str<strong>on</strong>g>focus</str<strong>on</strong>g>es <strong>on</strong> meeting the diverse needs <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
patients and their families and increasing the benefits that health care<br />
provides. We express this philosophy with the phrase “human health<br />
care,” and c<strong>on</strong>tinue to c<strong>on</strong>centrate efforts <strong>on</strong> its realizati<strong>on</strong> worldwide.<br />
Also embodied in our corporate philosophy is the belief that as a<br />
pharmaceutical manufacturer, our future depends <strong>on</strong> the ability to fill an<br />
essential role in the lives <str<strong>on</strong>g>of</str<strong>on</strong>g> patients and their families. To this end, we<br />
have defined our corporate objective as c<strong>on</strong>tributing to the quality <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
“human health care” under any health care system.<br />
Linked by our shared commitment to “human health care,” each<br />
employee at <str<strong>on</strong>g>Eisai</str<strong>on</strong>g> plays a key role in realizing this philosophy by c<strong>on</strong>stantly<br />
seeking ways to overcome the boundaries <str<strong>on</strong>g>of</str<strong>on</strong>g> nati<strong>on</strong>ality, time and system<br />
to provide relief to patients suffering from serious illnesses and peace <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
mind to their families. In order to make the benefits <str<strong>on</strong>g>of</str<strong>on</strong>g> safe and effective<br />
new drugs available to patients as rapidly as possible, we c<strong>on</strong>tinue to<br />
strengthen our global research and development, producti<strong>on</strong> and<br />
marketing activities.<br />
Results <str<strong>on</strong>g>of</str<strong>on</strong>g> Operati<strong>on</strong>s<br />
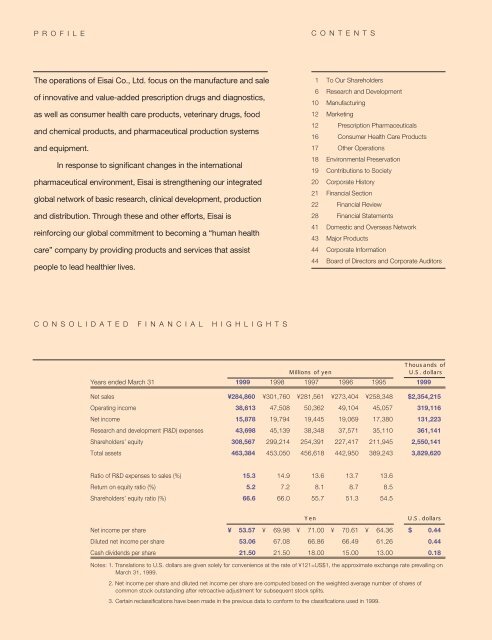
In fiscal 1998, which ended March 31, 1999, we recorded c<strong>on</strong>solidated<br />
net sales <str<strong>on</strong>g>of</str<strong>on</strong>g> ¥284.9 billi<strong>on</strong>, a decline <str<strong>on</strong>g>of</str<strong>on</strong>g> 5.6% from fiscal 1997. Operating<br />
income decreased 18.7%, to ¥38.6 billi<strong>on</strong>, while net income fell 19.8%,<br />
to ¥15.9 billi<strong>on</strong>.<br />
In the Pharmaceuticals segment, sales <str<strong>on</strong>g>of</str<strong>on</strong>g> Aricept, a novel treatment<br />
for Alzheimer’s disease, were ¥46.5 billi<strong>on</strong> in the United States and Europe,<br />
and sales <str<strong>on</strong>g>of</str<strong>on</strong>g> Pariet, a gastrointestinal disorder treatment, were ¥6.6<br />
billi<strong>on</strong> in Europe and Japan. <str<strong>on</strong>g>The</str<strong>on</strong>g>se c<strong>on</strong>tributed to total sales <str<strong>on</strong>g>of</str<strong>on</strong>g> ¥249.5<br />
billi<strong>on</strong>, which represents a 5.8% decline due to difficult market c<strong>on</strong>diti<strong>on</strong>s<br />
in Japan. Despite a good performance by the food additives and<br />
chemicals <str<strong>on</strong>g>operati<strong>on</strong>s</str<strong>on</strong>g>, sales in the Other segment declined 6.2%, to<br />
¥42.3 billi<strong>on</strong>, due to sluggish demand for pharmaceutical producti<strong>on</strong><br />
systems/equipment and animal health products.<br />
21
2<br />
Sales in the United States climbed 38.4%, to ¥57.4 billi<strong>on</strong>, allowing<br />
our U.S. <str<strong>on</strong>g>operati<strong>on</strong>s</str<strong>on</strong>g> to achieve pr<str<strong>on</strong>g>of</str<strong>on</strong>g>itability, while sales in Europe increased<br />
127.1%, to ¥10.2 billi<strong>on</strong>. In Japan, pharmaceutical manufacturers faced<br />
an increasingly harsh operating envir<strong>on</strong>ment, due to the third c<strong>on</strong>secutive<br />
year <str<strong>on</strong>g>of</str<strong>on</strong>g> a downward revisi<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>of</str<strong>on</strong>g>ficial prescripti<strong>on</strong> drug prices in fiscal<br />
1998 and the impact <str<strong>on</strong>g>of</str<strong>on</strong>g> an amendment to the Nati<strong>on</strong>al Health Insurance<br />
(NHI) Law in September 1997, which increased the porti<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> prescripti<strong>on</strong><br />
drug prices paid by individuals. Accordingly, domestic sales fell 11.2%,<br />
to ¥244.7 billi<strong>on</strong>. Elsewhere in Asia and in other markets, sales were<br />
down 18.1%, to ¥1.7 billi<strong>on</strong>, reflecting the impact <str<strong>on</strong>g>of</str<strong>on</strong>g> currency crises in<br />
Southeast Asia.<br />
Highlights <str<strong>on</strong>g>of</str<strong>on</strong>g> the Year<br />
<str<strong>on</strong>g>Eisai</str<strong>on</strong>g> c<strong>on</strong>tinues to <str<strong>on</strong>g>focus</str<strong>on</strong>g> <strong>on</strong> three essential objectives: further globalizati<strong>on</strong><br />
<str<strong>on</strong>g>of</str<strong>on</strong>g> our <str<strong>on</strong>g>operati<strong>on</strong>s</str<strong>on</strong>g>, c<strong>on</strong>tinued strengthening <str<strong>on</strong>g>of</str<strong>on</strong>g> R&D and the restructure<br />
<str<strong>on</strong>g>of</str<strong>on</strong>g> domestic <str<strong>on</strong>g>operati<strong>on</strong>s</str<strong>on</strong>g>. Our globalizati<strong>on</strong> efforts involve the growth <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
direct R&D, producti<strong>on</strong> and sales in key global markets. During the<br />
period under review, we substantially c<strong>on</strong>cluded an extensive capital<br />
investment program to establish facilities in the United States, Europe<br />
and Asia, and completed structural preparati<strong>on</strong>s to enable integrated<br />
R&D, producti<strong>on</strong> and sales in the United States. We expanded the<br />
sales <str<strong>on</strong>g>of</str<strong>on</strong>g> Aricept, our first prescripti<strong>on</strong> drug for the global market, making<br />
it available in a greater number <str<strong>on</strong>g>of</str<strong>on</strong>g> countries. We also launched Pariet,<br />
our sec<strong>on</strong>d major drug for the internati<strong>on</strong>al market, in Europe and<br />
Globalizing Operati<strong>on</strong>s<br />
Restructuring Domestic Operati<strong>on</strong>s<br />
Reinforcing R&D
Yuji Naito Haruo Naito<br />
Chairman President and Chief Executive Officer<br />
approval was received in the U.S. to market the drug under the trade<br />
name Aciphex. We expect to see str<strong>on</strong>g growth in sales <str<strong>on</strong>g>of</str<strong>on</strong>g> the two<br />
products, as well as in royalties from licensees and exports <str<strong>on</strong>g>of</str<strong>on</strong>g> drug<br />
substance/pharmaceutical bulk product.<br />
In the area <str<strong>on</strong>g>of</str<strong>on</strong>g> R&D, we c<strong>on</strong>tinued to bolster original drug research<br />
in Japan, the United States and Europe, aimed at developing our third<br />
and fourth global prescripti<strong>on</strong> drugs. In fiscal 1999, we will c<strong>on</strong>tinue<br />
to narrow the scope <str<strong>on</strong>g>of</str<strong>on</strong>g> our research, strengthen clinical development<br />
activities for new and existing candidates, and investigate promising<br />
new experimental compounds.<br />
Efforts to restructure our domestic <str<strong>on</strong>g>operati<strong>on</strong>s</str<strong>on</strong>g> centered <strong>on</strong> our pr<str<strong>on</strong>g>of</str<strong>on</strong>g>it<br />
base in the prescripti<strong>on</strong> pharmaceutical market. In April 1999, we<br />
3
4<br />
launched a significant restructuring <str<strong>on</strong>g>of</str<strong>on</strong>g> the administrative side <str<strong>on</strong>g>of</str<strong>on</strong>g> our<br />
domestic prescripti<strong>on</strong> pharmaceutical <str<strong>on</strong>g>operati<strong>on</strong>s</str<strong>on</strong>g>. This move will facilitate<br />
direct links between fr<strong>on</strong>t-line sales pers<strong>on</strong>nel and the head <str<strong>on</strong>g>of</str<strong>on</strong>g>fice,<br />
thereby ensuring our ability to provide the most appropriate informati<strong>on</strong><br />
for each marketing channel and to resp<strong>on</strong>d effectively and promptly<br />
to market needs. <str<strong>on</strong>g>The</str<strong>on</strong>g> restructuring will also enable us to better utilize<br />
domestic management resources to further globalize our <str<strong>on</strong>g>operati<strong>on</strong>s</str<strong>on</strong>g><br />
and reinforce R&D.<br />
<str<strong>on</strong>g>The</str<strong>on</strong>g>se three objectives will c<strong>on</strong>tinue to guide our efforts to positi<strong>on</strong><br />
<str<strong>on</strong>g>Eisai</str<strong>on</strong>g> as an R&D-driven global prescripti<strong>on</strong> pharmaceutical manufacturer.<br />
Realizing Global “Human Health Care"<br />
To realize our commitment to “human health care,” we are promoting<br />
what we have termed “knowledge-creati<strong>on</strong> activities.” Through<br />
“knowledge-creati<strong>on</strong> activities,” we cultivate an awareness <str<strong>on</strong>g>of</str<strong>on</strong>g> the needs<br />
<str<strong>on</strong>g>of</str<strong>on</strong>g> patients and c<strong>on</strong>sumers and share this knowledge am<strong>on</strong>g a wide<br />
spectrum <str<strong>on</strong>g>of</str<strong>on</strong>g> people inside and outside the <str<strong>on</strong>g>Co</str<strong>on</strong>g>mpany to facilitate the<br />
creati<strong>on</strong>, accumulati<strong>on</strong> and effective utilizati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> new ideas. We are<br />
c<strong>on</strong>fident that such activities will<br />
enable us to realize our missi<strong>on</strong><br />
to increase the benefits <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
health care to patients and<br />
c<strong>on</strong>sumers.<br />
<str<strong>on</strong>g>Eisai</str<strong>on</strong>g> currently employs more<br />
than 7,200 people worldwide,<br />
from a variety <str<strong>on</strong>g>of</str<strong>on</strong>g> cultural backgrounds<br />
and with c<strong>on</strong>trasting<br />
ideals and values. Despite<br />
differences in nati<strong>on</strong>ality, gender
and age, our shared dedicati<strong>on</strong> to “human health care” guides our<br />
efforts. In Japan, we have trained selected employees as “human<br />
health care” leaders through a program that allows them to gain<br />
experience by assisting health care pr<str<strong>on</strong>g>of</str<strong>on</strong>g>essi<strong>on</strong>als and interacting with<br />
patients in hospitals or other facilities. After completing the program,<br />
these employees return to their original workplace, where they are<br />
resp<strong>on</strong>sible for initiating and overseeing related projects. <str<strong>on</strong>g>The</str<strong>on</strong>g> “human<br />
health care” leaders also periodically participate in “human health care”<br />
c<strong>on</strong>ferences to present the results <str<strong>on</strong>g>of</str<strong>on</strong>g> their efforts, promote the sharing<br />
<str<strong>on</strong>g>of</str<strong>on</strong>g> informati<strong>on</strong> and experience and further cultivate leadership. By<br />
encouraging cooperati<strong>on</strong> am<strong>on</strong>g leaders worldwide, we will endeavor<br />
to promote systematized, global-level “knowledge-creati<strong>on</strong> activities.”<br />
Operating in an increasingly challenging envir<strong>on</strong>ment, characterized<br />
by growing pressure to lower health care costs, we will c<strong>on</strong>tinue to<br />
resp<strong>on</strong>d to health care needs worldwide by providing superior products<br />
and services. We look forward to the support <str<strong>on</strong>g>of</str<strong>on</strong>g> our shareholders as we<br />
proceed toward achievement <str<strong>on</strong>g>of</str<strong>on</strong>g> the “human health care” philosophy.<br />
Yuji Naito<br />
Chairman<br />
Haruo Naito<br />
President and Chief Executive Officer<br />
5
6<br />
Research and Development<br />
Creating an R&D Structure for the 21st Century<br />
We are currently taking steps to expand and strengthen our global R&D organizati<strong>on</strong>, which<br />
encompasses key facilities in Japan, the United States and Europe, to enhance our original<br />
drug development and clinical testing capabilities.<br />
<str<strong>on</strong>g>The</str<strong>on</strong>g> Tsukuba Research Laboratories in Japan c<strong>on</strong>centrate <strong>on</strong> four key therapeutic<br />
areas: inflammatory and allergic disorders; blood disorders, including complicati<strong>on</strong>s resulting<br />
from diabetes; cancer; and the central nervous system. <str<strong>on</strong>g>The</str<strong>on</strong>g> <str<strong>on</strong>g>Eisai</str<strong>on</strong>g> Research Institute <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
Bost<strong>on</strong>, Inc., specializes in cancer, inflammati<strong>on</strong>, immunological disorders and allergy as<br />
well as in advanced process research. In the United Kingdom, the <str<strong>on</strong>g>Eisai</str<strong>on</strong>g> L<strong>on</strong>d<strong>on</strong> Research<br />
Laboratories, <str<strong>on</strong>g>Ltd</str<strong>on</strong>g>. <str<strong>on</strong>g>focus</str<strong>on</strong>g> <strong>on</strong> basic molecular and cytological research aimed at formulating<br />
new c<strong>on</strong>cepts for the development <str<strong>on</strong>g>of</str<strong>on</strong>g> innovative drugs for diagnosing and treating cerebral<br />
and central nervous system disorders.<br />
To strengthen our global R&D activities, we restructured our R&D activities in August<br />
1998, bringing them under the direct c<strong>on</strong>trol <str<strong>on</strong>g>of</str<strong>on</strong>g> the president and placing senior executives<br />
in charge <str<strong>on</strong>g>of</str<strong>on</strong>g> basic research, product development and clinical testing.<br />
As in Japan, our R&D activities in the United States encompass basic research, pre-clinical<br />
and clinical testing, the development <str<strong>on</strong>g>of</str<strong>on</strong>g> pharmaceutical materials and process research.<br />
Our U.S. R&D facilities will thus play a crucial role in the globalizati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> our <str<strong>on</strong>g>operati<strong>on</strong>s</str<strong>on</strong>g>, and<br />
we will c<strong>on</strong>tinue to invest in them accordingly. In April 1999, we integrated R&D activities in<br />
the Bost<strong>on</strong> area by merging the <str<strong>on</strong>g>Eisai</str<strong>on</strong>g> Merrimack Valley Laboratories, Inc., where process<br />
and producti<strong>on</strong>-related research are c<strong>on</strong>ducted, with the <str<strong>on</strong>g>Eisai</str<strong>on</strong>g> Research Institute <str<strong>on</strong>g>of</str<strong>on</strong>g> Bost<strong>on</strong>,<br />
Inc. To improve the efficiency <str<strong>on</strong>g>of</str<strong>on</strong>g> these facilities, we will tightly <str<strong>on</strong>g>focus</str<strong>on</strong>g> their activities, prioritizing<br />
and accelerating the development <str<strong>on</strong>g>of</str<strong>on</strong>g> particularly promising new drug candidates. We<br />
will also explore the possibility <str<strong>on</strong>g>of</str<strong>on</strong>g> cooperative development, depending <strong>on</strong> the R&D theme,<br />
where such an approach promises to enhance results.<br />
Developing New Prescripti<strong>on</strong> Drugs for the Global Market<br />
<str<strong>on</strong>g>Eisai</str<strong>on</strong>g> invested ¥43.7 billi<strong>on</strong> in R&D in fiscal 1998, equivalent to 15.3% <str<strong>on</strong>g>of</str<strong>on</strong>g> c<strong>on</strong>solidated<br />
net sales. This is <strong>on</strong>e <str<strong>on</strong>g>of</str<strong>on</strong>g> the highest percentages am<strong>on</strong>g Japanese pharmaceutical<br />
manufacturers.<br />
R&D highlights during the period included applying for regulatory approval to manufacture<br />
and market Aricept in Japan as a treatment for Alzheimer’s disease. In the United States, we<br />
are clinically testing Aricept for additi<strong>on</strong>al indicati<strong>on</strong>s, namely for the treatment <str<strong>on</strong>g>of</str<strong>on</strong>g> dementia<br />
associated with cerebrovascular disease and attenti<strong>on</strong> deficit disorder/attenti<strong>on</strong> deficit hyperactivity<br />
disorder. We also are proceeding with clinical testing <str<strong>on</strong>g>of</str<strong>on</strong>g> the gastrointestinal disorder<br />
treatment Pariet. In additi<strong>on</strong> to receiving authorizati<strong>on</strong> to market Pariet in 15 countries in Europe<br />
during the period, we also received approval from the U.S. FDA in August 1999 to market<br />
the drug in the United States under the trade name Aciphex. <str<strong>on</strong>g>The</str<strong>on</strong>g> drug also holds potential as<br />
a treatment for Helicobacter pylori eradicati<strong>on</strong> and symptomatic reflux esophagitis, and we<br />
are currently supporting clinical development <str<strong>on</strong>g>of</str<strong>on</strong>g> the drug for expanded indicati<strong>on</strong>s.
8<br />
<str<strong>on</strong>g>The</str<strong>on</strong>g> ultimate objective <str<strong>on</strong>g>of</str<strong>on</strong>g> our R&D activities worldwide is to develop original prescripti<strong>on</strong><br />
drugs for the global market. Our researchers strive tirelessly in this quest to bring to market<br />
new drugs that increase the benefits that health care provides to patients worldwide.<br />
Promising research candidates at present include potential treatments for septic shock and<br />
cancer. We are also accelerating development efforts to introduce outstanding drugs from<br />
other manufacturers. In recent years, we c<strong>on</strong>cluded a license agreement with Bracco S.p.A<br />
to develop a new magnetic res<strong>on</strong>ance imaging (MRI) c<strong>on</strong>trast medium in Japan and entered<br />
into an agreement with Knoll AG to jointly develop a new obesity management drug for Japan.<br />
We also signed an agreement with Toyama Chemical <str<strong>on</strong>g>Co</str<strong>on</strong>g>., <str<strong>on</strong>g>Ltd</str<strong>on</strong>g>. <str<strong>on</strong>g>of</str<strong>on</strong>g> Japan to cooperate in the<br />
development and marketing <str<strong>on</strong>g>of</str<strong>on</strong>g> an exciting new anti-rheumatic agent.<br />
We will c<strong>on</strong>tinue to expand leading-edge R&D in key therapeutic areas and explore<br />
new directi<strong>on</strong>s in research to ensure our stature as a global manufacturer <str<strong>on</strong>g>of</str<strong>on</strong>g> prescripti<strong>on</strong><br />
pharmaceuticals and the future growth <str<strong>on</strong>g>of</str<strong>on</strong>g> our global <str<strong>on</strong>g>operati<strong>on</strong>s</str<strong>on</strong>g>.<br />
Improving the Efficiency <str<strong>on</strong>g>of</str<strong>on</strong>g> New Drug Development<br />
Developing a new drug requires a huge financial investment and a l<strong>on</strong>g period <str<strong>on</strong>g>of</str<strong>on</strong>g> time. In order for<br />
us to provide an effective new drug to patients as quickly as possible, we c<strong>on</strong>stantly endeavor to<br />
enhance the efficiency <str<strong>on</strong>g>of</str<strong>on</strong>g> pharmaceutical compound synthesis and screening, thereby shortening<br />
the development time. To help us achieve this goal, we are introducing various forms <str<strong>on</strong>g>of</str<strong>on</strong>g> automated<br />
systems at our R&D facilities worldwide.<br />
<str<strong>on</strong>g>Co</str<strong>on</strong>g>mbinatorial Chemistry<br />
<str<strong>on</strong>g>Co</str<strong>on</strong>g>mbinatorial chemistry is a new technology where a vast number <str<strong>on</strong>g>of</str<strong>on</strong>g> compounds having similar<br />
structure can be systematically prepared in a short period <str<strong>on</strong>g>of</str<strong>on</strong>g> time. This system efficiently produces<br />
compounds through the c<strong>on</strong>secutive reacti<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> chemical substances in soluti<strong>on</strong>s and <strong>on</strong> synthetic<br />
resin beads. This technology greatly improves the efficiency <str<strong>on</strong>g>of</str<strong>on</strong>g> synthesis, permitting a single<br />
researcher to synthesize approximately 100 to 200 compounds annually <strong>on</strong> average.<br />
High-Throughput Screening<br />
High-throughput screening utilizes an automated system that tests large<br />
numbers <str<strong>on</strong>g>of</str<strong>on</strong>g> synthetic and natural compounds for biological activity<br />
against new drug targets that have been identified at the molecular/<br />
cellular level. With the introducti<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> a screening robot, several milli<strong>on</strong><br />
compounds can be assayed in <strong>on</strong>e year, thus the search for a prototype<br />
compound, which is the basis for a new drug design, can be achieved<br />
efficiently.<br />
New Laboratory Established in Tsukuba<br />
In drug discovery it is increasingly important to find seeds for new drug<br />
development by <str<strong>on</strong>g>focus</str<strong>on</strong>g>ing <strong>on</strong> the disease mechanisms at the gene level.<br />
<str<strong>on</strong>g>The</str<strong>on</strong>g> recently established “Seeds Laboratory” in Tsukuba Research<br />
Laboratories investigates the functi<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> genes and their relati<strong>on</strong>ship<br />
to diseases from the perspective <str<strong>on</strong>g>of</str<strong>on</strong>g> molecular biology, genetics,<br />
bioinformatics and genome science in an attempt to identify new<br />
targets for drug development.
10<br />
Manufacturing<br />
Reinforcing Our Global Manufacturing Network<br />
Our network <str<strong>on</strong>g>of</str<strong>on</strong>g> producti<strong>on</strong> facilities in Japan, the United States and Europe promotes the<br />
local manufacture <str<strong>on</strong>g>of</str<strong>on</strong>g> products that have been developed internally.<br />
In the United States, we began <str<strong>on</strong>g>operati<strong>on</strong>s</str<strong>on</strong>g> at a new plant near Houst<strong>on</strong>, Texas, in June<br />
1998, enabling us to guarantee a stable supply <str<strong>on</strong>g>of</str<strong>on</strong>g> synthetic vitamin E. In November 1998,<br />
we commenced producti<strong>on</strong> and shipments <str<strong>on</strong>g>of</str<strong>on</strong>g> Aricept at a new plant in North Carolina. Our<br />
ability to satisfy the stringent regulati<strong>on</strong>s and envir<strong>on</strong>mental standards governing pharmaceutical<br />
plants in the United States is an important accomplishment in our drive to expand<br />
our global manufacturing network.<br />
In Asia, our plants in Bogor, Ind<strong>on</strong>esia, and Tainan, Taiwan, produce and repackage a<br />
variety <str<strong>on</strong>g>of</str<strong>on</strong>g> prescripti<strong>on</strong> pharmaceuticals, including Methycobal, a peripheral neuropathies<br />
treatment, and Neuquin<strong>on</strong>, a metabolic cardiot<strong>on</strong>ic. In May 1998, we completed c<strong>on</strong>structi<strong>on</strong><br />
<str<strong>on</strong>g>of</str<strong>on</strong>g> a plant in Suzhou, China, and began preparati<strong>on</strong>s to shift producti<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> drugs from<br />
the plant in Shenyang and to manufacture Aricept. <str<strong>on</strong>g>The</str<strong>on</strong>g> new plant will greatly enhance our<br />
ability to ensure a stable supply <str<strong>on</strong>g>of</str<strong>on</strong>g> pharmaceuticals in the Asian market.<br />
In Japan, the Kawashima and Misato plants are implementing measures in manufacturing<br />
and quality c<strong>on</strong>trol to ensure that we c<strong>on</strong>tinue to meet global standards <str<strong>on</strong>g>of</str<strong>on</strong>g> Good Manufacturing<br />
Practice (GMP), while improving producti<strong>on</strong> efficiency. <str<strong>on</strong>g>The</str<strong>on</strong>g> Kashima Plant works closely with<br />
our R&D facilities in Tsukuba, Bost<strong>on</strong> and L<strong>on</strong>d<strong>on</strong> to produce high-quality drug substance.<br />
U.S. Synthetic Vitamin E Plant Opens<br />
Since <str<strong>on</strong>g>Eisai</str<strong>on</strong>g>’s establishment, we have c<strong>on</strong>ducted integrated R&D, producti<strong>on</strong> and marketing <str<strong>on</strong>g>of</str<strong>on</strong>g> vitamin E<br />
in Japan, expanding producti<strong>on</strong> facilities at the Kawashima Plant in line with advancements in producti<strong>on</strong><br />
technology. Our new synthetic vitamin E plant near Houst<strong>on</strong>, Texas, was built to ensure our ability to<br />
guarantee a stable supply worldwide. With dual producti<strong>on</strong> in Japan and the United States, we are<br />
positi<strong>on</strong>ed well to resp<strong>on</strong>d to anticipated increases in demand.<br />
First U.S. Prescripti<strong>on</strong> Pharmaceutical Plant <str<strong>on</strong>g>Co</str<strong>on</strong>g>mpleted<br />
<str<strong>on</strong>g>The</str<strong>on</strong>g> opening <str<strong>on</strong>g>of</str<strong>on</strong>g> our new plant in North Carolina represents the culminati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> a l<strong>on</strong>g and successful effort,<br />
including meeting U.S. GMP standards, transferring producti<strong>on</strong> technology from Japan and applying to the<br />
U.S. Food and Drug Administrati<strong>on</strong> (FDA) for approval. In November 1998, the new facility began manufacturing<br />
Aricept. <str<strong>on</strong>g>The</str<strong>on</strong>g> plant is currently preparing to apply for approval to manufacture Aciphex in the United States<br />
(Pariet in Japan/Europe) as the transfer <str<strong>on</strong>g>of</str<strong>on</strong>g> producti<strong>on</strong> technology from Japan has been accomplished.<br />
Pharmaceutical Plant in China <str<strong>on</strong>g>Co</str<strong>on</strong>g>mpleted<br />
<str<strong>on</strong>g>Co</str<strong>on</strong>g>mpleted in 1998, the Suzhou plant has c<strong>on</strong>tinued to enhance manufacturing procedures, enabling it to<br />
meet China’s GMP standards, and has completed preparati<strong>on</strong>s to commence producti<strong>on</strong>. <str<strong>on</strong>g>The</str<strong>on</strong>g> plant will<br />
take over the manufacture <str<strong>on</strong>g>of</str<strong>on</strong>g> Neuquin<strong>on</strong> from the Shenyang plant, as well as the producti<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> several<br />
other drugs currently exported from Japan. In the near future, the plant will also begin producing Aricept.<br />
Repackaging <str<strong>on</strong>g>operati<strong>on</strong>s</str<strong>on</strong>g> began in 1999 and from 2000 the plant will actually commence producti<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
pharmaceuticals, thus becoming our premier supply base for prescripti<strong>on</strong> pharmaceuticals in Asia.<br />
Misato and Kashima Plants Achieve Internati<strong>on</strong>al Standards to Manufacture Drugs for<br />
Overseas Markets<br />
In January 1997, the Misato Plant was approved by the United Kingdom’s Medicines <str<strong>on</strong>g>Co</str<strong>on</strong>g>ntrol Agency (MCA),<br />
to begin producti<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> Pariet for the United Kingdom, Germany and other European markets. <str<strong>on</strong>g>The</str<strong>on</strong>g> Misato<br />
Plant also filed an applicati<strong>on</strong> with the U.S. FDA to produce Pariet (Aciphex in the United States) for the U.S.<br />
market. At the same time, measures have been taken to ensure the plants meet internati<strong>on</strong>al GMP standards<br />
<str<strong>on</strong>g>of</str<strong>on</strong>g> the U.S., Japan and Europe. In December 1998, the Misato Plant was audited and received FDA approval<br />
for the future producti<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> Aciphex. <str<strong>on</strong>g>The</str<strong>on</strong>g> Kashima Plant received FDA approval, following its successful<br />
applicati<strong>on</strong> to produce drug substance for Aricept in September 1996.
12<br />
Marketing—Prescripti<strong>on</strong> Pharmaceuticals<br />
In line with our “human health care” philosophy and our commitment to improving the<br />
benefits provided by heath care to patients and their families worldwide, we c<strong>on</strong>stantly<br />
work to expand our innovative and advanced pharmaceutical products and to provide<br />
accurate and up-to-date informati<strong>on</strong> <strong>on</strong> their use. Our two main drugs for the global market<br />
are Aricept and Pariet. In Japan, we <str<strong>on</strong>g>of</str<strong>on</strong>g>fer a broad range <str<strong>on</strong>g>of</str<strong>on</strong>g> products, the majority <str<strong>on</strong>g>of</str<strong>on</strong>g> which<br />
were developed in-house. <str<strong>on</strong>g>The</str<strong>on</strong>g>se encompass several key therapeutic and diagnostic areas,<br />
including digestive ailments and disorders <str<strong>on</strong>g>of</str<strong>on</strong>g> the nervous, muscular and cardiovascular<br />
systems. In all markets, we tailor our marketing activities to the needs <str<strong>on</strong>g>of</str<strong>on</strong>g> health care<br />
pr<str<strong>on</strong>g>of</str<strong>on</strong>g>essi<strong>on</strong>als and their patients.<br />
Expanding Global Efforts<br />
Reflecting <strong>on</strong>going efforts to strengthen our global, in-house marketing network, c<strong>on</strong>solidated<br />
overseas sales in fiscal 1998 rose 38.5% from the fiscal 1997 level. <str<strong>on</strong>g>The</str<strong>on</strong>g> primary factor<br />
behind this gain is the outstanding sales <str<strong>on</strong>g>of</str<strong>on</strong>g> Aricept during the period under review. Aricept<br />
is well-established as an Alzheimer’s disease treatment in key markets, which is underscored<br />
by the fact that Aricept is currently our top-selling prescripti<strong>on</strong> drug worldwide.<br />
In additi<strong>on</strong> to marketing through medical representatives in the United States, we also<br />
promote Aricept with a direct-to-c<strong>on</strong>sumer advertising campaign. We will c<strong>on</strong>tinue to<br />
market Aricept intensively to make this novel drug available to as many patients as possible.<br />
We also expect to begin sales so<strong>on</strong> for Aciphex in the United States (Pariet in Japan/Europe),<br />
which recently received marketing clearance.<br />
In Europe, sales <str<strong>on</strong>g>of</str<strong>on</strong>g> Aricept in the United Kingdom, Germany and France remain str<strong>on</strong>g.<br />
We received approval to market Pariet in the 15 countries <str<strong>on</strong>g>of</str<strong>on</strong>g> the European Uni<strong>on</strong> in 1998,<br />
launching sales in the United Kingdom and Germany shortly thereafter.<br />
Marketing activities in Asia date back to the 1960s. Our product line in the regi<strong>on</strong> currently<br />
includes Methycobal, a peripheral neuropathies treatment, and My<strong>on</strong>al, a muscle relaxant.<br />
In fiscal 1998, we launched Aricept in Thailand, H<strong>on</strong>g K<strong>on</strong>g and several other key markets<br />
in the regi<strong>on</strong>. Reflecting intense marketing efforts, sales <str<strong>on</strong>g>of</str<strong>on</strong>g> Aricept in Asia c<strong>on</strong>tinue to grow<br />
steadily. We have received approvals for Pariet in H<strong>on</strong>g K<strong>on</strong>g and the Philippines, and plan<br />
to introduce Pariet in Asia at the earliest possible date.
14<br />
Reinforcing Domestic Capabilities<br />
Our principal products in Japan are Selbex, a gastritis/gastric ulcer medicati<strong>on</strong>;<br />
Methycobal, a peripheral neuropathies treatment; Iomer<strong>on</strong>, a n<strong>on</strong>-i<strong>on</strong>ic c<strong>on</strong>trast medium;<br />
Glakay, an osteoporosis treatment; and the global product Pariet.<br />
Selbex is currently the top gastritis/gastric ulcer medicati<strong>on</strong> <strong>on</strong> the market in Japan,<br />
and in October 1998, <str<strong>on</strong>g>Eisai</str<strong>on</strong>g> received a patent for gastritis treatment for Selbex in Japan. On<br />
another fr<strong>on</strong>t, 30-day prescripti<strong>on</strong>s for Glakay received <str<strong>on</strong>g>of</str<strong>on</strong>g>ficial approval, a positive development<br />
that has reduced the frequency with which osteoporosis sufferers must obtain new<br />
prescripti<strong>on</strong>s. Pariet’s efficacy in treating duodenal peptic ulcers has also earned it a str<strong>on</strong>g<br />
following am<strong>on</strong>g gastroenterologists and patients in the domestic market.<br />
Improving Informati<strong>on</strong> Services<br />
To ensure our ability to provide our customers with accurate and timely informati<strong>on</strong> <strong>on</strong> our<br />
products, we c<strong>on</strong>stantly work to enhance academic and practical training for our medical<br />
representatives. Again, our commitment to global “human health care” is evident. <str<strong>on</strong>g>The</str<strong>on</strong>g> job<br />
<str<strong>on</strong>g>of</str<strong>on</strong>g> an <str<strong>on</strong>g>Eisai</str<strong>on</strong>g> medical representative is to advise fr<strong>on</strong>t-line medical pr<str<strong>on</strong>g>of</str<strong>on</strong>g>essi<strong>on</strong>als <strong>on</strong> pharmaceuticals<br />
and treatment methods. Accordingly, medical representatives frequently participate<br />
in seminars and practical training sessi<strong>on</strong>s to update their knowledge and skills. Given the<br />
increasing emphasis <strong>on</strong> evidence-based medicine in hospitals, we will c<strong>on</strong>tinue to place a<br />
high priority <strong>on</strong> the provisi<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> quality informati<strong>on</strong>, as well as <strong>on</strong> the training <str<strong>on</strong>g>of</str<strong>on</strong>g> medical<br />
representatives, thereby earning the trust and respect <str<strong>on</strong>g>of</str<strong>on</strong>g> health care pr<str<strong>on</strong>g>of</str<strong>on</strong>g>essi<strong>on</strong>als and<br />
patients.<br />
Domestic Marketing Activities Restructured<br />
To cope with changes in Japan’s health care system, we restructured our domestic prescripti<strong>on</strong><br />
pharmaceutical marketing activities in April 1999 to enhance our resp<strong>on</strong>siveness to the diverse needs<br />
<str<strong>on</strong>g>of</str<strong>on</strong>g> special-purpose hospitals, regular hospitals, clinics and dispensing pharmacies. This restructuring<br />
also involved creating direct links between medical representatives and the head <str<strong>on</strong>g>of</str<strong>on</strong>g>fice, ensuring our<br />
ability to provide the most appropriate informati<strong>on</strong> for each type <str<strong>on</strong>g>of</str<strong>on</strong>g> facility.<br />
New Products Launched<br />
In June 1998, we launched Cleactor, a revoluti<strong>on</strong>ary, fast-acting thrombolytic for treatment <str<strong>on</strong>g>of</str<strong>on</strong>g> acute<br />
myocardial infarcti<strong>on</strong>. In November 1998, we marketed Tambocor Injecti<strong>on</strong>, an anti-arrhythmic agent<br />
for hospital usage. Due to their outstanding efficacy in treating patients needing acute care, these<br />
drugs are expected to attract significant demand in the future.
Aricept<br />
Aricept is a reversible acetylcholinesterase inhibitor<br />
for the treatment <str<strong>on</strong>g>of</str<strong>on</strong>g> Alzheimer’s disease. It has<br />
obtained approval in numerous countries worldwide.<br />
By inhibiting the acti<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> acetylcholinesterase—<br />
the enzyme that breaks down the neurotransmitter<br />
acetylcholine—this drug increases the c<strong>on</strong>centrati<strong>on</strong><br />
<str<strong>on</strong>g>of</str<strong>on</strong>g> acetylcholine in the brain, improving cognitive<br />
functi<strong>on</strong>, providing symptomatic relief for patients<br />
suffering from mild to moderate Alzheimer’s disease.<br />
Aricept, which requires administrati<strong>on</strong> <strong>on</strong>ly <strong>on</strong>ce<br />
daily, represents a significant step forward in the<br />
treatment <str<strong>on</strong>g>of</str<strong>on</strong>g> Alzheimer’s disease and <str<strong>on</strong>g>of</str<strong>on</strong>g>fers new<br />
hope for Alzheimer’s suffers and their caregivers.<br />
Pariet<br />
Pariet, a prot<strong>on</strong> pump inhibitor, inhibits the secreti<strong>on</strong><br />
<str<strong>on</strong>g>of</str<strong>on</strong>g> gastric acid by blocking the functi<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> the enzyme<br />
resp<strong>on</strong>sible at the final stage <str<strong>on</strong>g>of</str<strong>on</strong>g> secreti<strong>on</strong>. <str<strong>on</strong>g>The</str<strong>on</strong>g> drug,<br />
which is administered <strong>on</strong>ce daily, has proven highly<br />
effective in relieving stomach pain and healing gastric<br />
ulcers, duodenal ulcers and gastroesophageal reflux<br />
disease.<br />
While H2 receptor antag<strong>on</strong>ists c<strong>on</strong>tinue to account<br />
for the largest share <str<strong>on</strong>g>of</str<strong>on</strong>g> the market for gastritis and<br />
gastric ulcer treatments, prot<strong>on</strong> pump inhibitors are<br />
attracting increasing attenti<strong>on</strong>. We have positi<strong>on</strong>ed<br />
Pariet as our sec<strong>on</strong>d major drug for the global market<br />
following Aricept and look forward to broadening its<br />
availability in the years ahead.<br />
15
16<br />
Marketing—<str<strong>on</strong>g>Co</str<strong>on</strong>g>nsumer Health Care Products<br />
Emphasizing Brands<br />
<str<strong>on</strong>g>The</str<strong>on</strong>g> c<strong>on</strong>sumer health care segment encompasses a wide range <str<strong>on</strong>g>of</str<strong>on</strong>g> items sold over the<br />
counter (OTC) at drugstores and pharmacies, including n<strong>on</strong>-prescripti<strong>on</strong> pharmaceuticals<br />
and medicated topical treatments, developed by <str<strong>on</strong>g>Eisai</str<strong>on</strong>g> in resp<strong>on</strong>se to the needs <str<strong>on</strong>g>of</str<strong>on</strong>g> healthc<strong>on</strong>scious<br />
c<strong>on</strong>sumers.<br />
Principal products in this segment, such as vitamin B2 preparati<strong>on</strong> Chocola BB and<br />
Sacl<strong>on</strong>, a treatment for indigesti<strong>on</strong> and heartburn c<strong>on</strong>taining chlorophyll, have been favored<br />
by customers for decades. We also market a number <str<strong>on</strong>g>of</str<strong>on</strong>g> “switch-OTC” products, n<strong>on</strong>prescripti<strong>on</strong><br />
drugs that began as prescripti<strong>on</strong> pharmaceuticals and have been reformulated<br />
and reclassified after extensive use has verified their safety. <str<strong>on</strong>g>The</str<strong>on</strong>g>se include the OTC mainstay<br />
Nabolin, an active-type B12 preparati<strong>on</strong> that relieves numbness, stiffness and discomfort in<br />
the shoulder and lumbar area caused by peripheral nerve damage.<br />
Since <str<strong>on</strong>g>Eisai</str<strong>on</strong>g>’s establishment, we have been <strong>on</strong> the forefr<strong>on</strong>t <str<strong>on</strong>g>of</str<strong>on</strong>g> vitamin E research, and<br />
products include the Juvelux series <str<strong>on</strong>g>of</str<strong>on</strong>g> natural vitamin E preparati<strong>on</strong>s, popular as an effective<br />
preventative measure against a variety <str<strong>on</strong>g>of</str<strong>on</strong>g> ailments, including neck and shoulder stiffness<br />
and numbness <str<strong>on</strong>g>of</str<strong>on</strong>g> the extremities. In August 1998, we introduced Chocola EC, a natural<br />
vitamin E and C preparati<strong>on</strong> that fades liver spots and freckles, as well as relieves sensitivity<br />
to cold in the hands and feet and stiff shoulders. <str<strong>on</strong>g>The</str<strong>on</strong>g> product has proved to be particularly<br />
popular am<strong>on</strong>g women in all age groups.<br />
We c<strong>on</strong>tinue to <str<strong>on</strong>g>focus</str<strong>on</strong>g> <strong>on</strong> expanding our OTC <str<strong>on</strong>g>operati<strong>on</strong>s</str<strong>on</strong>g> by emphasizing brands suited<br />
to the OTC market and reinforcing brand awareness. On the development fr<strong>on</strong>t, we are<br />
c<strong>on</strong>centrating <strong>on</strong> products that maximize our accumulated expertise and capabilities in the<br />
c<strong>on</strong>sumer health care area<br />
New Products Launched<br />
Additi<strong>on</strong>s to the <str<strong>on</strong>g>Eisai</str<strong>on</strong>g> OTC product line in fiscal 1998 include Chocola EC, a natural vitamin E and C<br />
preparati<strong>on</strong> that fades liver spots and freckles, as well as relieves sensitivity to cold in the hands and<br />
feet and stiff shoulders. In May 1999, we launched Sea-b<strong>on</strong>d, a sea kelp-derived natural sheet-type<br />
denture adhesive. Because Sea-b<strong>on</strong>d is a sheet-type denture adhesive, it provides a uniform<br />
adhesi<strong>on</strong> and is easily removed.<br />
Marketing Strategies Emphasize Product Characteristics<br />
Marketing strategies for OTC products c<strong>on</strong>tinue to <str<strong>on</strong>g>focus</str<strong>on</strong>g> <strong>on</strong> publicizing unique product characteristics.<br />
For products such as Juvelux and Nabolin, we <str<strong>on</strong>g>focus</str<strong>on</strong>g> promoti<strong>on</strong> efforts <strong>on</strong> providing informati<strong>on</strong><br />
about correct product usage through seminars and study sessi<strong>on</strong>s for pharmacists and drugstore<br />
operators. For marketing <str<strong>on</strong>g>of</str<strong>on</strong>g> products like Chocola BB and Sacl<strong>on</strong>, we c<strong>on</strong>centrate <strong>on</strong> advertising in<br />
a variety <str<strong>on</strong>g>of</str<strong>on</strong>g> media. In 1998, we expanded these efforts to include Internet banner advertisements.
Marketing—Other Operati<strong>on</strong>s<br />
Food Additives and Chemicals Operati<strong>on</strong>s<br />
To ensure tastier and safer food products through the use <str<strong>on</strong>g>of</str<strong>on</strong>g> natural materials,<br />
<str<strong>on</strong>g>Eisai</str<strong>on</strong>g> has for many years c<strong>on</strong>ducted extensive R&D to develop food additives<br />
based <strong>on</strong> vitamin E. Mainstay products in this category include vitamin E-enriched<br />
supplements and antioxidants, as well as food preservatives, sweeteners and<br />
flavor enhancers, which we supply to manufacturers <str<strong>on</strong>g>of</str<strong>on</strong>g> food products. Our<br />
chemicals <str<strong>on</strong>g>operati<strong>on</strong>s</str<strong>on</strong>g> center <strong>on</strong> natural and synthetic vitamin E, and also include<br />
pharmaceutical raw materials, such as lysozyme hydrochloride.<br />
To ensure a stable supply <str<strong>on</strong>g>of</str<strong>on</strong>g> these products and improve our resp<strong>on</strong>siveness<br />
to the needs <str<strong>on</strong>g>of</str<strong>on</strong>g> customers worldwide, we commenced <str<strong>on</strong>g>operati<strong>on</strong>s</str<strong>on</strong>g> in 1998 at<br />
a new synthetic vitamin E plant in the U.S.<br />
Veterinary and Livestock Product Operati<strong>on</strong>s<br />
Our <str<strong>on</strong>g>operati<strong>on</strong>s</str<strong>on</strong>g> in this category are guided by the c<strong>on</strong>cept that the health <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
humans and animals are closely intertwined. In additi<strong>on</strong> to developing an extensive<br />
selecti<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> products in the areas <str<strong>on</strong>g>of</str<strong>on</strong>g> nutriti<strong>on</strong>, preventative hygiene and disease<br />
treatment, we have <str<strong>on</strong>g>focus</str<strong>on</strong>g>ed <strong>on</strong> resp<strong>on</strong>ding to market needs by developing highly<br />
competitive products and strengthening our marketing capabilities.<br />
We also market a broad range <str<strong>on</strong>g>of</str<strong>on</strong>g> vitamin E-based nutriti<strong>on</strong>al supplements<br />
and livestock feeds, as well as swine vaccines and other immunological products,<br />
disinfectants for envir<strong>on</strong>mental sterilizati<strong>on</strong>, antibiotic and antibacterial agents for<br />
treating bacterial diseases, and veterinary drugs for pets. <str<strong>on</strong>g>The</str<strong>on</strong>g>se products are<br />
available in Japan, many other Asian countries and Oceania.<br />
Pharmaceutical Producti<strong>on</strong> Systems and Equipment<br />
Operati<strong>on</strong>s in this category endeavor to provide pharmaceutical manufacturers<br />
worldwide with pharmaceutical producti<strong>on</strong> systems and equipment that ensure<br />
quality by <str<strong>on</strong>g>focus</str<strong>on</strong>g>ing <strong>on</strong> instruments used to detect impurities in ampules. We have<br />
sold globally more than 700 units <str<strong>on</strong>g>of</str<strong>on</strong>g> our Automatic Inspecti<strong>on</strong> Machine (AIM), a<br />
high-precisi<strong>on</strong> instrument that enables highly sophisticated detecti<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> impurities in<br />
ampules. Other achievements include the High Frequency Wave Sterilizati<strong>on</strong> System<br />
(MWS), a c<strong>on</strong>tinuous sterilizati<strong>on</strong> system, and the <str<strong>on</strong>g>Eisai</str<strong>on</strong>g> Inspecti<strong>on</strong> System (EIS), a<br />
comprehensive testing system. In 1998, we launched a technologically advanced,<br />
fully automated testing system named NAIM.<br />
17
18<br />
Envir<strong>on</strong>mental Preservati<strong>on</strong><br />
To ensure our ability to functi<strong>on</strong> in harm<strong>on</strong>y with nature and fulfill the role society expects,<br />
we c<strong>on</strong>fr<strong>on</strong>t envir<strong>on</strong>mental issues in all areas <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>Co</str<strong>on</strong>g>mpany activity ranging from R&D and<br />
producti<strong>on</strong> to marketing.<br />
We have already met domestic pharmaceutical industry targets for the year 2000 in<br />
terms <str<strong>on</strong>g>of</str<strong>on</strong>g> waste output, recycling, external emissi<strong>on</strong>s and materials disposal, and we<br />
c<strong>on</strong>tinue to work to improve our record. We have also set targets for reducing resource<br />
and energy c<strong>on</strong>sumpti<strong>on</strong> and are taking decisive steps to ensure these targets are met.<br />
In February 1999, the Kawashima Plant received ISO 14001 certificati<strong>on</strong>, the Internati<strong>on</strong>al<br />
Organizati<strong>on</strong> for Standardizati<strong>on</strong>’s benchmark for envir<strong>on</strong>mental management systems,<br />
for drug substance producti<strong>on</strong> and drug formulati<strong>on</strong> <str<strong>on</strong>g>operati<strong>on</strong>s</str<strong>on</strong>g>. <str<strong>on</strong>g>The</str<strong>on</strong>g> Misato and Kashima<br />
plants are currently proceeding with preparati<strong>on</strong>s to apply for ISO 14001 certificati<strong>on</strong>.<br />
We believe we have a global resp<strong>on</strong>sibility to protect the natural envir<strong>on</strong>ment and<br />
c<strong>on</strong>serve precious resources for subsequent generati<strong>on</strong>s. This commitment will c<strong>on</strong>tinue to<br />
guide our envir<strong>on</strong>mental preservati<strong>on</strong> efforts in all <str<strong>on</strong>g>of</str<strong>on</strong>g> our <str<strong>on</strong>g>operati<strong>on</strong>s</str<strong>on</strong>g>.<br />
Envir<strong>on</strong>mentally Friendly Packaging<br />
Methycobal/<br />
Peripheral neuropathies treatment<br />
Iomer<strong>on</strong>/<br />
N<strong>on</strong>-i<strong>on</strong>ic c<strong>on</strong>trast medium<br />
Kurtime/<br />
Bath additive c<strong>on</strong>taining fermented<br />
rice extract<br />
Skainar Rhinitis S/<br />
Sinusitis relief medicati<strong>on</strong><br />
Skainar Antitussive and Expectorant/<br />
<str<strong>on</strong>g>Co</str<strong>on</strong>g>ugh and cold medicati<strong>on</strong><br />
Sacl<strong>on</strong> Q/<br />
Indigesti<strong>on</strong> and heartburn relief<br />
Tablets are packed in Light Protect Easy (LPE), a proprietary<br />
packaging that is safer to use and easily disposable.<br />
Packaging comp<strong>on</strong>ents can be easily separated prior to<br />
disposal without special equipment.<br />
Use <str<strong>on</strong>g>of</str<strong>on</strong>g> a laminated bottle reduces toxic gas emissi<strong>on</strong>s when<br />
incinerated.<br />
Polypropylene has replaced polyvinyl chloride (PVC) in<br />
packaging.<br />
PTP (Press Through Pack) packaging c<strong>on</strong>tains <strong>on</strong>ly<br />
polypropylene materials.
26<br />
Total current liabilities as <str<strong>on</strong>g>of</str<strong>on</strong>g> March 31, 1999, amounted to ¥87,311 milli<strong>on</strong>, up ¥11,564 milli<strong>on</strong>,<br />
or 15.3%. This increase largely reflects the shift <str<strong>on</strong>g>of</str<strong>on</strong>g> ¥10,000 milli<strong>on</strong> in b<strong>on</strong>ds from l<strong>on</strong>g-term liabilities<br />
to the current liabilities. <str<strong>on</strong>g>Co</str<strong>on</strong>g>nsequently, total l<strong>on</strong>g-term liabilities declined ¥8,965 milli<strong>on</strong>, or 13.1%,<br />
to ¥59,259 milli<strong>on</strong>.<br />
Total shareholder’s equity rose ¥9,353 milli<strong>on</strong>, or 3.1%, to ¥308,567 milli<strong>on</strong>, reflecting additi<strong>on</strong>s<br />
from net income and the payment <str<strong>on</strong>g>of</str<strong>on</strong>g> dividends and b<strong>on</strong>uses to directors. <str<strong>on</strong>g>The</str<strong>on</strong>g> shareholders’ equity<br />
ratio rose to 66.6%, from 66.0% a year earlier.<br />
Cash Flows<br />
Net cash provided by operating activities amounted to ¥35,886 milli<strong>on</strong>, up ¥11,469 milli<strong>on</strong> from<br />
the previous period. Despite declines in net income and gains <strong>on</strong> sales <str<strong>on</strong>g>of</str<strong>on</strong>g> short-term investments<br />
and investment securities from the previous year <str<strong>on</strong>g>of</str<strong>on</strong>g> ¥3,916 milli<strong>on</strong> and ¥2,307 milli<strong>on</strong>, respectively,<br />
purchases <str<strong>on</strong>g>of</str<strong>on</strong>g> property, plant and equipment in recent years resulted in a ¥2,111 milli<strong>on</strong> increase in<br />
depreciati<strong>on</strong> and amortizati<strong>on</strong>, and total changes in assets and liability related <str<strong>on</strong>g>operati<strong>on</strong>s</str<strong>on</strong>g> yielded<br />
an increase <str<strong>on</strong>g>of</str<strong>on</strong>g> ¥14,479 milli<strong>on</strong>.<br />
Net cash used in investing activities rose ¥17,701 milli<strong>on</strong>, to ¥31,173 milli<strong>on</strong>. Purchases <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
property, plant and equipment decreased ¥16,652 milli<strong>on</strong>. Purchases <str<strong>on</strong>g>of</str<strong>on</strong>g> short-term investments<br />
and investment securities rose ¥41,223 milli<strong>on</strong>.<br />
Net cash used in financing activities declined ¥16,278 milli<strong>on</strong>, to ¥10,018 milli<strong>on</strong>. Of the total,<br />
¥1,642 represented payments <strong>on</strong> bank borrowings, while ¥6,373 milli<strong>on</strong> was applied to dividends<br />
paid. Because no straight or c<strong>on</strong>vertible b<strong>on</strong>ds were redeemed during the period, repayment <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
l<strong>on</strong>g-term debt declined ¥19,576 milli<strong>on</strong>, to ¥2,138 milli<strong>on</strong>.<br />
As a c<strong>on</strong>sequence <str<strong>on</strong>g>of</str<strong>on</strong>g> the <str<strong>on</strong>g>Co</str<strong>on</strong>g>mpany’s operating, investing and financing activities during the<br />
period, and the effect <str<strong>on</strong>g>of</str<strong>on</strong>g> exchange rate changes, cash and cash equivalents <strong>on</strong> March 31, 1999,<br />
totaled ¥47,279 milli<strong>on</strong>, down ¥6,295 milli<strong>on</strong> from ¥53,574 milli<strong>on</strong> <strong>on</strong> March 31, 1998.
Year 2000 <str<strong>on</strong>g>Co</str<strong>on</strong>g>mpliance<br />
<str<strong>on</strong>g>Eisai</str<strong>on</strong>g> recognizes the Year 2000 compliance as a key management issue and is taking appropriate<br />
steps not <strong>on</strong>ly to achieve technical compliance, but also to protect ordinary business <str<strong>on</strong>g>operati<strong>on</strong>s</str<strong>on</strong>g>.<br />
In fiscal 1997, <str<strong>on</strong>g>Eisai</str<strong>on</strong>g> established a Year 2000 issue project team to guide companywide efforts<br />
to address the issue. <str<strong>on</strong>g>The</str<strong>on</strong>g> project team has taken the lead in analyzing potential risks, not <strong>on</strong>ly for<br />
the <str<strong>on</strong>g>Co</str<strong>on</strong>g>mpany’s internal systems, but also for those <str<strong>on</strong>g>of</str<strong>on</strong>g> business partners and customers. Details<br />
<str<strong>on</strong>g>of</str<strong>on</strong>g> compliance efforts and their implementati<strong>on</strong> are m<strong>on</strong>itored by the <str<strong>on</strong>g>Co</str<strong>on</strong>g>mpany’s internal audit<br />
secti<strong>on</strong> and reported regularly to the Management <str<strong>on</strong>g>Co</str<strong>on</strong>g>mmittee.<br />
Testing <str<strong>on</strong>g>of</str<strong>on</strong>g> the <str<strong>on</strong>g>Co</str<strong>on</strong>g>mpany’s mainframe computer systems, which c<strong>on</strong>stitute the nucleus <str<strong>on</strong>g>of</str<strong>on</strong>g> the<br />
basic operating system, were completed in March 1999. <str<strong>on</strong>g>Eisai</str<strong>on</strong>g> expects to complete countermeasures<br />
for servers, pers<strong>on</strong>al computers and networks in September 1999. Inspecti<strong>on</strong>s <str<strong>on</strong>g>of</str<strong>on</strong>g> process c<strong>on</strong>trol<br />
systems and equipment and facilities c<strong>on</strong>taining microchips are currently underway and are<br />
scheduled for completi<strong>on</strong> in September 1999.<br />
A <str<strong>on</strong>g>Co</str<strong>on</strong>g>ntingency Planning project team has been organized to identify and address latent risks<br />
associated with the <str<strong>on</strong>g>Co</str<strong>on</strong>g>mpany’s systems and those <str<strong>on</strong>g>of</str<strong>on</strong>g> its business partners and customers, as<br />
well as infrastructure-related risks.<br />
Although a rati<strong>on</strong>al plan has been developed to manage Year 2000 compliance, the <str<strong>on</strong>g>Co</str<strong>on</strong>g>mpany<br />
cannot completely guarantee the eliminati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> all risks associated with this issue.<br />
27
EISAI CO., LTD. AND CONSOLIDATED SUBSIDIARIES<br />
N O T E S T O C O N S O L I D A T E D F I N A N C I A L S T A T E M E N T S<br />
Years ended March 31, 1999 and 1998<br />
NOTE 1.<br />
Basis <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
Presenting<br />
<str<strong>on</strong>g>Co</str<strong>on</strong>g>nsolidated<br />
Financial<br />
Statements<br />
NOTE 2.<br />
Significant<br />
Accounting<br />
Policies<br />
<str<strong>on</strong>g>The</str<strong>on</strong>g> accompanying c<strong>on</strong>solidated financial statements <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>Eisai</str<strong>on</strong>g> <str<strong>on</strong>g>Co</str<strong>on</strong>g>., <str<strong>on</strong>g>Ltd</str<strong>on</strong>g>. (the “<str<strong>on</strong>g>Co</str<strong>on</strong>g>mpany”), and its c<strong>on</strong>solidated subsidiaries<br />
have been prepared from the c<strong>on</strong>solidated financial statements issued for domestic reporting purposes in accordance with<br />
the provisi<strong>on</strong>s set forth in the Japanese Securities and Exchange Law and its related accounting regulati<strong>on</strong>s. <str<strong>on</strong>g>The</str<strong>on</strong>g> <str<strong>on</strong>g>Co</str<strong>on</strong>g>mpany<br />
and its domestic c<strong>on</strong>solidated subsidiaries maintain their accounts and records in c<strong>on</strong>formity with accounting principles and<br />
practices generally accepted in Japan which are different in certain respects as to applicati<strong>on</strong> and disclosure requirements <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
Internati<strong>on</strong>al Accounting Standards, and its foreign c<strong>on</strong>solidated subsidiaries in c<strong>on</strong>formity with those <str<strong>on</strong>g>of</str<strong>on</strong>g> their domicile. <str<strong>on</strong>g>The</str<strong>on</strong>g><br />
c<strong>on</strong>solidated financial statements are not intended to present the financial positi<strong>on</strong>, result <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>operati<strong>on</strong>s</str<strong>on</strong>g> and cash flows in<br />
accordance with accounting principles and practices generally accepted in countries and jurisdicti<strong>on</strong>s other than Japan.<br />
In preparing the c<strong>on</strong>solidated financial statements, certain reclassificati<strong>on</strong>s and rearrangements have been made to the<br />
c<strong>on</strong>solidated financial statements issued domestically in order to present them in a form which is more familiar to readers<br />
outside Japan. <str<strong>on</strong>g>The</str<strong>on</strong>g> c<strong>on</strong>solidated statements <str<strong>on</strong>g>of</str<strong>on</strong>g> cash flows are not required as a part <str<strong>on</strong>g>of</str<strong>on</strong>g> the basic c<strong>on</strong>solidated financial<br />
statements under Japanese laws and regulati<strong>on</strong>s but are presented herein as additi<strong>on</strong>al informati<strong>on</strong>. In additi<strong>on</strong>, the<br />
accompanying notes include informati<strong>on</strong> which is not required under accounting principles and practices generally accepted<br />
in Japan but is presented as additi<strong>on</strong>al informati<strong>on</strong>.<br />
<str<strong>on</strong>g>The</str<strong>on</strong>g> c<strong>on</strong>solidated financial statements are stated in Japanese yen, the currency <str<strong>on</strong>g>of</str<strong>on</strong>g> the country in which the <str<strong>on</strong>g>Co</str<strong>on</strong>g>mpany<br />
principally operates. <str<strong>on</strong>g>The</str<strong>on</strong>g> United States dollar amounts included herein are given solely for c<strong>on</strong>venience <str<strong>on</strong>g>of</str<strong>on</strong>g> readers outside<br />
Japan and have been translated, as a matter <str<strong>on</strong>g>of</str<strong>on</strong>g> arithmetical computati<strong>on</strong> <strong>on</strong>ly, at the rate <str<strong>on</strong>g>of</str<strong>on</strong>g> ¥121=US$1, the approximate<br />
exchange rate at March 31, 1999. <str<strong>on</strong>g>The</str<strong>on</strong>g> United States dollar amounts should not be c<strong>on</strong>strued as a representati<strong>on</strong> that<br />
the Japanese yen amounts have been, or could in the future be, c<strong>on</strong>verted into United States dollars at the above or any<br />
other rate.<br />
Certain reclassificati<strong>on</strong>s have been made in the 1998 c<strong>on</strong>solidated financial statements to c<strong>on</strong>form to the classificati<strong>on</strong>s<br />
used in 1999. <str<strong>on</strong>g>The</str<strong>on</strong>g>se reclassificati<strong>on</strong>s had no effect <strong>on</strong> previously reported net income or retained earnings.<br />
(a) Principles <str<strong>on</strong>g>of</str<strong>on</strong>g> c<strong>on</strong>solidati<strong>on</strong><br />
<str<strong>on</strong>g>The</str<strong>on</strong>g> accompanying c<strong>on</strong>solidated financial statements include the accounts <str<strong>on</strong>g>of</str<strong>on</strong>g> the <str<strong>on</strong>g>Co</str<strong>on</strong>g>mpany and substantially all <str<strong>on</strong>g>of</str<strong>on</strong>g> its<br />
subsidiaries. All significant intercompany accounts and transacti<strong>on</strong>s have been eliminated in c<strong>on</strong>solidati<strong>on</strong>. All material<br />
unrealized pr<str<strong>on</strong>g>of</str<strong>on</strong>g>it included in assets resulting from transacti<strong>on</strong>s within the <str<strong>on</strong>g>Co</str<strong>on</strong>g>mpany and its c<strong>on</strong>solidated subsidiaries has<br />
also been eliminated.<br />
<str<strong>on</strong>g>The</str<strong>on</strong>g> excess cost <str<strong>on</strong>g>of</str<strong>on</strong>g> investments in subsidiaries over the equity in net assets at acquisiti<strong>on</strong> dates is being amortized<br />
over five years using the straight-line method.<br />
(b) Cash and cash equivalents<br />
For purposes <str<strong>on</strong>g>of</str<strong>on</strong>g> the statements <str<strong>on</strong>g>of</str<strong>on</strong>g> cash flows, the <str<strong>on</strong>g>Co</str<strong>on</strong>g>mpany c<strong>on</strong>siders all time deposits to be cash equivalents. Time<br />
deposits have original maturities <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>on</strong>e year or less and can be withdrawn <strong>on</strong> demand with no diminuti<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> principal.<br />
(c) Short-term investments and investment securities<br />
Listed securities including short-term investments and investment securities are mainly valued at the lower <str<strong>on</strong>g>of</str<strong>on</strong>g> cost or market.<br />
Other securities are stated at cost. <str<strong>on</strong>g>The</str<strong>on</strong>g> cost <str<strong>on</strong>g>of</str<strong>on</strong>g> securities sold is mainly determined using the moving-average-cost method.<br />
Investments in unc<strong>on</strong>solidated subsidiaries and associated companies (20% to 50% ownership) are accounted for by<br />
the equity method.<br />
(d) Inventories<br />
Inventories are generally stated at weighted average cost.<br />
(e) Property, plant and equipment<br />
Property, plant and equipment are stated at cost. Depreciati<strong>on</strong> is computed using the declining balance method at rates<br />
based <strong>on</strong> the estimated useful lives <str<strong>on</strong>g>of</str<strong>on</strong>g> the assets.<br />
In 1998, the estimated useful lives <str<strong>on</strong>g>of</str<strong>on</strong>g> property, plant and equipment other than buildings and structures <str<strong>on</strong>g>of</str<strong>on</strong>g> the head <str<strong>on</strong>g>of</str<strong>on</strong>g>fice,<br />
branches, and laboratories <str<strong>on</strong>g>of</str<strong>on</strong>g> the <str<strong>on</strong>g>Co</str<strong>on</strong>g>mpany, and buildings and structures <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>Eisai</str<strong>on</strong>g> Chemical <str<strong>on</strong>g>Co</str<strong>on</strong>g>., <str<strong>on</strong>g>Ltd</str<strong>on</strong>g>., which was merged into<br />
the <str<strong>on</strong>g>Co</str<strong>on</strong>g>mpany as <str<strong>on</strong>g>of</str<strong>on</strong>g> April 1, 1998, were changed to be duplicative as l<strong>on</strong>g as those used in prior years. This change was made<br />
because it was determined that the buildings and structures <str<strong>on</strong>g>of</str<strong>on</strong>g> plants acquired had l<strong>on</strong>ger lives resulting from refurbishment<br />
and repair to such machinery and equipment. <str<strong>on</strong>g>The</str<strong>on</strong>g> effect <str<strong>on</strong>g>of</str<strong>on</strong>g> this change for the year ended March 31, 1998, was to decrease<br />
depreciati<strong>on</strong> by ¥4,075 milli<strong>on</strong> and increase operating income, and income before income taxes by ¥3,305 milli<strong>on</strong>.<br />
(f) Leases<br />
Leases are accounted for as operating leases. Under Japanese accounting standards for leases, finance leases that deem to<br />
transfer ownership <str<strong>on</strong>g>of</str<strong>on</strong>g> the leased property to the lessee are to be capitalized, while other finance leases are permitted to be<br />
accounted for as operating lease transacti<strong>on</strong>s if certain “as if capitalized” informati<strong>on</strong> is disclosed in the notes to the lessee’s<br />
financial statements.<br />
33
34<br />
(g) Income taxes<br />
<str<strong>on</strong>g>The</str<strong>on</strong>g> <str<strong>on</strong>g>Co</str<strong>on</strong>g>mpany and domestic subsidiaries provide for income taxes at the amount currently payable for each year. <str<strong>on</strong>g>The</str<strong>on</strong>g> tax<br />
effect <str<strong>on</strong>g>of</str<strong>on</strong>g> temporary differences between tax and financial reporting purposes is not recorded. Certain overseas subsidiaries<br />
provide for deferred income taxes relating to temporary differences in accordance with accounting principles generally<br />
accepted in the relevant countries.<br />
(h) Retirement allowances<br />
<str<strong>on</strong>g>The</str<strong>on</strong>g> annual provisi<strong>on</strong> for retirement allowances is calculated to present the liability for employees principally at the amount<br />
that would be required if all employees voluntarily terminated their employment at each balance sheet date, less amounts<br />
funded by a c<strong>on</strong>tributory pensi<strong>on</strong> plan disregarding a porti<strong>on</strong> specified by government regulati<strong>on</strong>s and for directors and<br />
corporate auditors at the amount that would be required if all directors and corporate auditors retired at each balance sheet date.<br />
Pensi<strong>on</strong> costs, including prior service costs, which are amortized principally over 20 years, are charged to income when<br />
c<strong>on</strong>tributed to the fund.<br />
(i) Appropriati<strong>on</strong>s <str<strong>on</strong>g>of</str<strong>on</strong>g> retained earnings<br />
Appropriati<strong>on</strong>s <str<strong>on</strong>g>of</str<strong>on</strong>g> retained earnings at each year-end are reflected in the financial statements for the following year up<strong>on</strong><br />
shareholders’ approval.<br />
(j) Research and development expenses<br />
Research and development expenses are charged to income as incurred.<br />
(k) Deferred charges<br />
Stock issuance costs are charged to income as incurred. Start up costs related to certain subsidiaries are deferred and<br />
amortized by the straight-line method over four to five years.<br />
(l) Foreign currency transacti<strong>on</strong>s<br />
Short-term receivables and payables denominated in foreign currencies are translated into Japanese yen at the current<br />
exchange rates at each balance sheet date.<br />
L<strong>on</strong>g-term receivables and payables denominated in foreign currencies are generally translated into Japanese yen<br />
at historical exchange rates.<br />
(m) Foreign currency financial statements<br />
<str<strong>on</strong>g>The</str<strong>on</strong>g> balance sheet accounts <str<strong>on</strong>g>of</str<strong>on</strong>g> the c<strong>on</strong>solidated overseas subsidiaries are translated into yen at the current exchange<br />
rates as <str<strong>on</strong>g>of</str<strong>on</strong>g> the balance sheet date except for shareholders’ equity, which is translated at the historical exchange rate.<br />
Differences arising from such translati<strong>on</strong> are shown as “Currency translati<strong>on</strong> adjustments” in the accompanying <str<strong>on</strong>g>Co</str<strong>on</strong>g>nsolidated<br />
Balance Sheets. Revenue and expense accounts <str<strong>on</strong>g>of</str<strong>on</strong>g> the c<strong>on</strong>solidated overseas subsidiaries are translated into yen at the<br />
average rates.<br />
(n) Per share informati<strong>on</strong><br />
<str<strong>on</strong>g>The</str<strong>on</strong>g> computati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> net income per share is based <strong>on</strong> the weighted average number <str<strong>on</strong>g>of</str<strong>on</strong>g> shares <str<strong>on</strong>g>of</str<strong>on</strong>g> comm<strong>on</strong> stock outstanding<br />
during each year, retroactively adjusted for stock splits. <str<strong>on</strong>g>The</str<strong>on</strong>g> average number <str<strong>on</strong>g>of</str<strong>on</strong>g> comm<strong>on</strong> shares used in the computati<strong>on</strong> was<br />
296,405,452 shares for 1999 and 282,846,622 shares for 1998.<br />
Diluted net income per share <str<strong>on</strong>g>of</str<strong>on</strong>g> comm<strong>on</strong> stock assumes full c<strong>on</strong>versi<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> the outstanding c<strong>on</strong>vertible b<strong>on</strong>ds at the<br />
beginning <str<strong>on</strong>g>of</str<strong>on</strong>g> the year with an applicable adjustment for related interest expense, net <str<strong>on</strong>g>of</str<strong>on</strong>g> tax.<br />
Cash dividends per share presented in the accompanying <str<strong>on</strong>g>Co</str<strong>on</strong>g>nsolidated Statements <str<strong>on</strong>g>of</str<strong>on</strong>g> Income are dividends applicable<br />
to the respective years without making retroactive adjustment for subsequent stock splits.
40<br />
I N D E P E N D E N T A U D I T O R S ’ R E P O R T<br />
To the Board <str<strong>on</strong>g>of</str<strong>on</strong>g> Directors and Shareholders <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>Eisai</str<strong>on</strong>g> <str<strong>on</strong>g>Co</str<strong>on</strong>g>., <str<strong>on</strong>g>Ltd</str<strong>on</strong>g>.<br />
We have examined the c<strong>on</strong>solidated balance sheets <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>Eisai</str<strong>on</strong>g> <str<strong>on</strong>g>Co</str<strong>on</strong>g>., <str<strong>on</strong>g>Ltd</str<strong>on</strong>g>. and c<strong>on</strong>solidated subsidiaries as <str<strong>on</strong>g>of</str<strong>on</strong>g> March 31, 1999<br />
and 1998, and the related c<strong>on</strong>solidated statements <str<strong>on</strong>g>of</str<strong>on</strong>g> income, shareholders’ equity, and cash flows for the years then ended,<br />
all expressed in Japanese yen. Our examinati<strong>on</strong>s were made in accordance with auditing standards, procedures and<br />
practices generally accepted and applied in Japan and, accordingly, included such tests <str<strong>on</strong>g>of</str<strong>on</strong>g> the accounting records and such<br />
other auditing procedures as we c<strong>on</strong>sidered necessary in the circumstances.<br />
In our opini<strong>on</strong>, the c<strong>on</strong>solidated financial statements referred to above present fairly the financial positi<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>Eisai</str<strong>on</strong>g> <str<strong>on</strong>g>Co</str<strong>on</strong>g>., <str<strong>on</strong>g>Ltd</str<strong>on</strong>g>.<br />
and c<strong>on</strong>solidated subsidiaries as <str<strong>on</strong>g>of</str<strong>on</strong>g> March 31, 1999 and 1998, and the results <str<strong>on</strong>g>of</str<strong>on</strong>g> their <str<strong>on</strong>g>operati<strong>on</strong>s</str<strong>on</strong>g> and their cash flows for the<br />
years then ended in c<strong>on</strong>formity with accounting principles and practices generally accepted in Japan applied <strong>on</strong> a c<strong>on</strong>sistent basis.<br />
Our examinati<strong>on</strong>s also comprehended the translati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> Japanese yen amounts into U.S. dollar amounts and, in our<br />
opini<strong>on</strong>, such translati<strong>on</strong> has been made in c<strong>on</strong>formity with the basis stated in Note 1 to the c<strong>on</strong>solidated financial statements.<br />
Such U.S. dollar amounts are presented solely for the c<strong>on</strong>venience <str<strong>on</strong>g>of</str<strong>on</strong>g> readers outside Japan.<br />
Tokyo, Japan<br />
June 29, 1999
DOMESTIC AND OVERSEAS NETWORK<br />
Domestic Network<br />
Head Office<br />
4-6-10 Koishikawa, Bunkyo-ku,<br />
Tokyo 112-8088, Japan<br />
Tel: 03-3817-3700<br />
Support Centers<br />
Hokkaido Support Center<br />
Tohoku Support Center<br />
Tokyo Support Center<br />
Tokai-Hokuriku Support Center<br />
Kansai Support Center<br />
Chu-Shikoku Support Center<br />
Kyushyu Support Center<br />
Producti<strong>on</strong> Facilities<br />
Misato Plant<br />
Kawashima Plant<br />
Kashima Plant<br />
Research Laboratory<br />
Tsukuba Research Laboratories<br />
Major Subsidiaries<br />
Japan<br />
Sanko Junyaku <str<strong>on</strong>g>Co</str<strong>on</strong>g>., <str<strong>on</strong>g>Ltd</str<strong>on</strong>g>.<br />
Chiyoda-ku, Tokyo<br />
Tel: 03-3863-3271 Fax: 03-3864-5644<br />
Sannova <str<strong>on</strong>g>Co</str<strong>on</strong>g>., <str<strong>on</strong>g>Ltd</str<strong>on</strong>g>.*<br />
Nitta-gun, Gunma<br />
Tel: 0276-52-3611 Fax: 0276-52-1341<br />
Elmed <str<strong>on</strong>g>Eisai</str<strong>on</strong>g> <str<strong>on</strong>g>Co</str<strong>on</strong>g>., <str<strong>on</strong>g>Ltd</str<strong>on</strong>g>.<br />
Toshima-ku, Tokyo<br />
Tel: 03-3980-6633 Fax: 03-3980-6634<br />
Eland <str<strong>on</strong>g>Co</str<strong>on</strong>g>., <str<strong>on</strong>g>Ltd</str<strong>on</strong>g>.<br />
Bunkyo-ku, Tokyo<br />
Tel: 03-3817-3987 Fax: 03-3811-7581<br />
<str<strong>on</strong>g>Eisai</str<strong>on</strong>g> Distributi<strong>on</strong> <str<strong>on</strong>g>Co</str<strong>on</strong>g>., <str<strong>on</strong>g>Ltd</str<strong>on</strong>g>.<br />
Atsugi-shi, Kanagawa<br />
Tel: 046-248-2655 Fax: 046-248-5909<br />
Kan Research Institute, Inc.<br />
Shimogyo-ku, Kyoto<br />
Tel: 075-325-5118 Fax: 075-325-5130<br />
Clinical Supply <str<strong>on</strong>g>Co</str<strong>on</strong>g>., <str<strong>on</strong>g>Ltd</str<strong>on</strong>g>.<br />
Hashima-gun, Gifu<br />
Tel: 058689-2712 Fax: 058689-3225<br />
Takehaya <str<strong>on</strong>g>Co</str<strong>on</strong>g>., <str<strong>on</strong>g>Ltd</str<strong>on</strong>g>.<br />
Bunkyo-ku, Tokyo<br />
Tel: 03-3817-5340 Fax: 03-3811-0216<br />
Herusu <str<strong>on</strong>g>Co</str<strong>on</strong>g>., <str<strong>on</strong>g>Ltd</str<strong>on</strong>g>.<br />
Toshima-ku, Tokyo<br />
Tel: 03-3971-4919 Fax: 03-3971-5346<br />
<str<strong>on</strong>g>Eisai</str<strong>on</strong>g> Seikaken <str<strong>on</strong>g>Co</str<strong>on</strong>g>., <str<strong>on</strong>g>Ltd</str<strong>on</strong>g>.<br />
Bunkyo-ku, Tokyo<br />
Tel: 03-5689-6460 Fax: 03-5689-6464<br />
Kawashima <str<strong>on</strong>g>Co</str<strong>on</strong>g>., <str<strong>on</strong>g>Ltd</str<strong>on</strong>g>.<br />
Hashima-gun, Gifu<br />
Tel: 058689-2881 Fax: 058689-3691<br />
Dymec <str<strong>on</strong>g>Co</str<strong>on</strong>g>., <str<strong>on</strong>g>Ltd</str<strong>on</strong>g>.<br />
Ichikawa-shi, Chiba<br />
Tel: 047-396-1611 Fax: 047-396-1600<br />
Hisakata <str<strong>on</strong>g>Co</str<strong>on</strong>g>., <str<strong>on</strong>g>Ltd</str<strong>on</strong>g>.<br />
Bunkyo-ku, Tokyo<br />
Tel: 03-3813-6898 Fax: 03-3818-9940<br />
Seiansha <str<strong>on</strong>g>Co</str<strong>on</strong>g>., <str<strong>on</strong>g>Ltd</str<strong>on</strong>g>.<br />
Toshima-ku, Tokyo<br />
Tel: 03-3980-1021 Fax: 03-3980-7302<br />
Bracco-<str<strong>on</strong>g>Eisai</str<strong>on</strong>g> <str<strong>on</strong>g>Co</str<strong>on</strong>g>., <str<strong>on</strong>g>Ltd</str<strong>on</strong>g>.<br />
Bunkyo-ku, Tokyo<br />
Tel: 03-3813-4311 Fax: 03-3813-4348<br />
Gakuen Shoji <str<strong>on</strong>g>Co</str<strong>on</strong>g>., <str<strong>on</strong>g>Ltd</str<strong>on</strong>g>.<br />
Tsukuba-shi, Ibaraki<br />
Tel: 0298-47-8422 Fax: 0298-47-5039<br />
* In August 1999, Sansho <str<strong>on</strong>g>Co</str<strong>on</strong>g>., <str<strong>on</strong>g>Ltd</str<strong>on</strong>g>. was renamed Sannova <str<strong>on</strong>g>Co</str<strong>on</strong>g>., <str<strong>on</strong>g>Ltd</str<strong>on</strong>g>.<br />
41
42<br />
North America<br />
<str<strong>on</strong>g>Eisai</str<strong>on</strong>g> <str<strong>on</strong>g>Co</str<strong>on</strong>g>rporati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> North America<br />
Glenpointe Centre West, 5th Floor<br />
500 Frank W. Burr Boulevard<br />
Teaneck, New Jersey 07666, U.S.A.<br />
Tel: 1-201-692-1100 Fax: 1-201-692-1804<br />
<str<strong>on</strong>g>Eisai</str<strong>on</strong>g> Research Institute <str<strong>on</strong>g>of</str<strong>on</strong>g> Bost<strong>on</strong>, Inc.*<br />
4 <str<strong>on</strong>g>Co</str<strong>on</strong>g>rporate Drive<br />
Andover, Massachusetts 01810-2441, U.S.A.<br />
Tel: 1-978-794-1117 Fax: 1-978-794-4910<br />
<str<strong>on</strong>g>Eisai</str<strong>on</strong>g> U.S.A., Inc.<br />
Marath<strong>on</strong> Oil Tower, Suite 690<br />
5555 San Felipe Road<br />
Houst<strong>on</strong>, Texas 77056, U.S.A.<br />
Tel: 1-713-621-9030 Fax: 1-713-621-9095<br />
<str<strong>on</strong>g>Eisai</str<strong>on</strong>g> Inc.<br />
Glenpointe Centre West, 5th Floor<br />
500 Frank W. Burr Boulevard<br />
Teaneck, New Jersey 07666, U.S.A.<br />
Tel: 1-201-692-1100 Fax: 1-201-692-1804<br />
Europe<br />
<str<strong>on</strong>g>Eisai</str<strong>on</strong>g> L<strong>on</strong>d<strong>on</strong> Research Laboratories, <str<strong>on</strong>g>Ltd</str<strong>on</strong>g>.<br />
Bernard Katz Building, University <str<strong>on</strong>g>Co</str<strong>on</strong>g>llege L<strong>on</strong>d<strong>on</strong><br />
Gower Street, L<strong>on</strong>d<strong>on</strong> WC1E 6BT, U.K.<br />
Tel: 44-171-388-4746 Fax: 44-171-413-1121<br />
<str<strong>on</strong>g>Eisai</str<strong>on</strong>g> <str<strong>on</strong>g>Ltd</str<strong>on</strong>g>.<br />
Hammersmith Internati<strong>on</strong>al Centre, 3 Shortlands, 2nd Floor<br />
L<strong>on</strong>d<strong>on</strong> W6 8EE, U.K.<br />
Tel: 44-181-600-1400 Fax: 44-181-600-1401<br />
<str<strong>on</strong>g>Eisai</str<strong>on</strong>g> Pharma-Chem Europe <str<strong>on</strong>g>Ltd</str<strong>on</strong>g>.<br />
<str<strong>on</strong>g>Co</str<strong>on</strong>g>mm<strong>on</strong>wealth House, Hammersmith Internati<strong>on</strong>al Centre<br />
2 Chalkhill Road, L<strong>on</strong>d<strong>on</strong> W6 8DW, U.K.<br />
Tel: 44-181-741-1330 Fax: 44-181-913-0019<br />
<str<strong>on</strong>g>Eisai</str<strong>on</strong>g> <strong>GmbH</strong><br />
Ly<strong>on</strong>er Strasse 14<br />
D-60528 Frankfurt am Main, Germany<br />
Tel: 49-69-665850 Fax: 49-69-6658525<br />
<str<strong>on</strong>g>Eisai</str<strong>on</strong>g> Machinery <strong>GmbH</strong><br />
Mathias Brueggen Strasse 142<br />
D-50829 <str<strong>on</strong>g>Co</str<strong>on</strong>g>logne, Germany<br />
Tel: 49-221-9564590, Fax: 49-221-9564599<br />
<str<strong>on</strong>g>Eisai</str<strong>on</strong>g>-Novartis <strong>GmbH</strong> & <str<strong>on</strong>g>Co</str<strong>on</strong>g>. KG<br />
Hochstrasse 3-5<br />
D-90429 Nuremberg, Germany<br />
Tel: 49-911-27312240 Fax: 49-911-27312816<br />
<str<strong>on</strong>g>Eisai</str<strong>on</strong>g> S.A.<br />
Tour Manhattan, 5-6 Place de I’Iris<br />
92095 Paris La Défense 2 cedex, France<br />
Tel: 33-1-47670005 Fax: 33-1-47670015<br />
<str<strong>on</strong>g>Eisai</str<strong>on</strong>g> B.V.<br />
Strawinskylaan 909, 1077 XX<br />
Amsterdam, <str<strong>on</strong>g>The</str<strong>on</strong>g> Netherlands<br />
Tel: 31-20-575-3340 Fax: 31-20-575-3341<br />
* In April 1999, <str<strong>on</strong>g>Eisai</str<strong>on</strong>g> Merrimack Valley Laboratories, Inc. merged with <str<strong>on</strong>g>Eisai</str<strong>on</strong>g> Research Institute<br />
<str<strong>on</strong>g>of</str<strong>on</strong>g> Bost<strong>on</strong>, Inc.<br />
Asia<br />
P.T. <str<strong>on</strong>g>Eisai</str<strong>on</strong>g> Ind<strong>on</strong>esia<br />
New Summitmas ll 12th Floor Jl, Jend. Sudirman Kav. 61-62<br />
Jakarta 12069, Ind<strong>on</strong>esia<br />
Tel: 62-21-522-6780 Fax: 62-21-522-6790<br />
<str<strong>on</strong>g>Eisai</str<strong>on</strong>g> Asia Regi<strong>on</strong>al Services Pte. <str<strong>on</strong>g>Ltd</str<strong>on</strong>g>.<br />
152 Beach Road No. 11-04<br />
Gateway East, Singapore 0718<br />
Tel: 65-296-6977 Fax: 65-292-2185<br />
<str<strong>on</strong>g>Eisai</str<strong>on</strong>g> (Malaysia) Sdn. Bhd.<br />
74, Jalan University, 46200 Petaling Jaya<br />
Selangor, Malaysia<br />
Tel: 60-3-757-6964 Fax: 60-3-757-9211<br />
<str<strong>on</strong>g>Eisai</str<strong>on</strong>g> (Thailand) Marketing <str<strong>on</strong>g>Co</str<strong>on</strong>g>., <str<strong>on</strong>g>Ltd</str<strong>on</strong>g>.<br />
6th Floor, Diethelm Tower A, 93/1 Wireless Road<br />
Bangkok 10330, Thailand<br />
Tel: 66-2-256-6296 Fax: 66-2-256-6299<br />
Hi-<str<strong>on</strong>g>Eisai</str<strong>on</strong>g> Pharmaceutical Inc.<br />
4th Floor, Reliable Building, 7230 Malugay Street<br />
Makati, Metro Manila, Philippines<br />
Tel: 63-2-893-9636 Fax: 63-2-818-7745<br />
<str<strong>on</strong>g>Eisai</str<strong>on</strong>g> H<strong>on</strong>g K<strong>on</strong>g <str<strong>on</strong>g>Co</str<strong>on</strong>g>., <str<strong>on</strong>g>Ltd</str<strong>on</strong>g>.<br />
Room 307, Carnival <str<strong>on</strong>g>Co</str<strong>on</strong>g>mmercial Centre<br />
18 Java Road, North Point, H<strong>on</strong>g K<strong>on</strong>g, China<br />
Tel: 852-2516-6128 Fax: 852-2561-5042<br />
<str<strong>on</strong>g>Eisai</str<strong>on</strong>g> Korea Inc.<br />
16th Floor Textile Center, 944-31, Daechi-3 d<strong>on</strong>g<br />
Kangnam-ku, Seoul 135-283, Republic <str<strong>on</strong>g>of</str<strong>on</strong>g> Korea<br />
Tel: 82-2-528-0555 Fax: 82-2-528-0557<br />
<str<strong>on</strong>g>Eisai</str<strong>on</strong>g> Taiwan, Inc.<br />
9th Floor, No. 18, Chang An E. Road, Sec. 1<br />
Taipei, Taiwan<br />
Tel: 886-2-2-531-4175 Fax: 886-2-2-531-0063<br />
Weizai <str<strong>on</strong>g>Co</str<strong>on</strong>g>., <str<strong>on</strong>g>Ltd</str<strong>on</strong>g>.<br />
9th Floor, No. 18, Chang An E. Road, Sec. 1<br />
Taipei, Taiwan<br />
Tel: 886-2-2-531-4175 Fax: 886-2-2-531-0063<br />
<str<strong>on</strong>g>Eisai</str<strong>on</strong>g> (Suzhou) Pharmaceutical <str<strong>on</strong>g>Co</str<strong>on</strong>g>., <str<strong>on</strong>g>Ltd</str<strong>on</strong>g>.<br />
Bai Yu Road #32 Suzhou Industrial Park<br />
Suzhou, Jiangsu Province, 215021, China<br />
Tel: 86512-761-3211 Fax: 86512-761-8640<br />
Shenyang <str<strong>on</strong>g>Eisai</str<strong>on</strong>g> Pharmaceutical <str<strong>on</strong>g>Co</str<strong>on</strong>g>., <str<strong>on</strong>g>Ltd</str<strong>on</strong>g>.<br />
No. 47 Bai Ta Lu Da D<strong>on</strong>g<br />
District Shenyang, 110041, China<br />
Tel: 8624-8852-0516 Fax: 8624-8850-0734
44<br />
CORPORATE INFORMATION<br />
(As <str<strong>on</strong>g>of</str<strong>on</strong>g> March 31, 1999)<br />
Date <str<strong>on</strong>g>of</str<strong>on</strong>g> Foundati<strong>on</strong>:<br />
1941<br />
<str<strong>on</strong>g>Co</str<strong>on</strong>g>rporate Address:<br />
<str<strong>on</strong>g>Eisai</str<strong>on</strong>g> <str<strong>on</strong>g>Co</str<strong>on</strong>g>., <str<strong>on</strong>g>Ltd</str<strong>on</strong>g>.<br />
4-6-10 Koishikawa, Bunkyo-ku,<br />
Tokyo 112-8088, Japan<br />
Tel: 03-3817-3700<br />
Annual Meeting:<br />
<str<strong>on</strong>g>The</str<strong>on</strong>g> annual shareholders’ meeting <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>Eisai</str<strong>on</strong>g> <str<strong>on</strong>g>Co</str<strong>on</strong>g>., <str<strong>on</strong>g>Ltd</str<strong>on</strong>g>., is held<br />
in June.<br />
Stock Exchange Listings:<br />
<str<strong>on</strong>g>Eisai</str<strong>on</strong>g> comm<strong>on</strong> stock is listed <strong>on</strong> the Tokyo Stock Exchange<br />
and the Osaka Securities Exchange<br />
Independent Public Accountants:<br />
Deloitte Touche Tohmatsu (by Tohmatsu & <str<strong>on</strong>g>Co</str<strong>on</strong>g>., the Japanese<br />
member firm <str<strong>on</strong>g>of</str<strong>on</strong>g> Deloitte Touche Tohmatsu Internati<strong>on</strong>al)<br />
MS Shibaura Bldg. 4-13-23, Shibaura,<br />
Minato-ku, Tokyo 108-0023, Japan<br />
Paid-in Capital:<br />
¥44,853 milli<strong>on</strong><br />
Number <str<strong>on</strong>g>of</str<strong>on</strong>g> Shares Outstanding:<br />
296,414,231<br />
Number <str<strong>on</strong>g>of</str<strong>on</strong>g> Shareholders:<br />
22,637<br />
Transfer Agent:<br />
<str<strong>on</strong>g>The</str<strong>on</strong>g> Toyo Trust and Banking <str<strong>on</strong>g>Co</str<strong>on</strong>g>., <str<strong>on</strong>g>Ltd</str<strong>on</strong>g>.<br />
Depositary and Agent with Respect to American Depositary<br />
Receipts for <str<strong>on</strong>g>Co</str<strong>on</strong>g>mm<strong>on</strong> Shares:<br />
Morgan Guaranty Trust <str<strong>on</strong>g>Co</str<strong>on</strong>g>mpany <str<strong>on</strong>g>of</str<strong>on</strong>g> New York<br />
60 Wall Street,<br />
New York, New York 10260-0060, U.S.A.<br />
Newspaper for Public Notice:<br />
Nih<strong>on</strong> Keizai Shimbun<br />
BOARD OF DIRECTORS AND CORPORATE AUDITORS<br />
(As <str<strong>on</strong>g>of</str<strong>on</strong>g> June 29, 1999)<br />
Chairman<br />
Yuji Naito<br />
President and Chief Executive Officer<br />
Haruo Naito<br />
Executive Vice President<br />
Yoshito Kishi<br />
Senior Vice President<br />
Hiromasa Nakai<br />
Managing Directors<br />
Koichi Ogawa<br />
Soichi Matsuno<br />
Aishin Shinoda<br />
Tatsuo Komaki<br />
Tadashi Nakai<br />
Ichiro Shinkai<br />
Directors<br />
Yukio Akimoto<br />
Hiroshi Yamauchi<br />
Nobukatsu Hashimoto<br />
Isao Okabayashi<br />
Teruo Osawa<br />
Mitsuhiro Ebata<br />
Koichi Katayama<br />
Yoji Takaoka<br />
Matsuo Ohara<br />
Jiro Hasegawa<br />
Hideaki Matsui<br />
Kenji Toda<br />
<str<strong>on</strong>g>Co</str<strong>on</strong>g>rporate Auditors<br />
Masato Sasaki<br />
Nobuo Eda<br />
Kimihiko Hiyama<br />
Shin Okada