Solubility, Ksp Worksheet 1
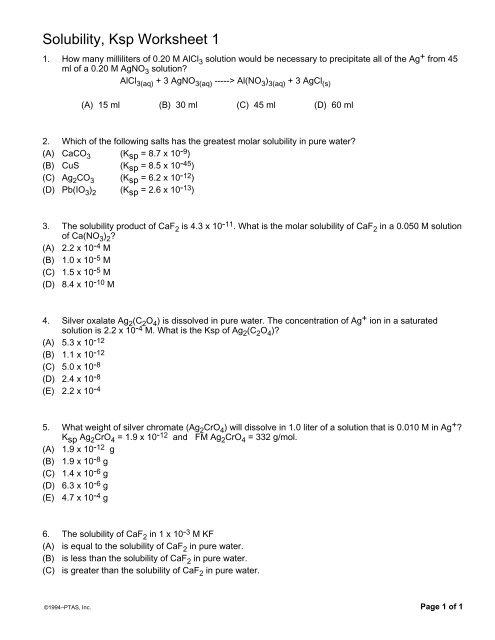
Solubility, Ksp Worksheet 1
Solubility, Ksp Worksheet 1
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Solubility</strong>, <strong>Ksp</strong> <strong>Worksheet</strong> 1<br />
1. How many milliliters of 0.20 M AlCl 3 solution would be necessary to precipitate all of the Ag + from 45<br />
ml of a 0.20 M AgNO 3 solution?<br />
AlCl 3(aq) + 3 AgNO 3(aq) -----> Al(NO 3 ) 3(aq) + 3 AgCl (s)<br />
(A) 15 ml (B) 30 ml (C) 45 ml (D) 60 ml<br />
2. Which of the following salts has the greatest molar solubility in pure water?<br />
(A) CaCO 3 (K sp = 8.7 x 10 -9 )<br />
(B) CuS (K sp = 8.5 x 10 -45 )<br />
(C) Ag 2 CO 3 (K sp = 6.2 x 10 -12 )<br />
(D) Pb(IO 3 ) 2 (K sp = 2.6 x 10 -13 )<br />
3. The solubility product of CaF 2 is 4.3 x 10 -11 . What is the molar solubility of CaF 2 in a 0.050 M solution<br />
of Ca(NO 3 ) 2 ?<br />
(A) 2.2 x 10 -4 M<br />
(B) 1.0 x 10 -5 M<br />
(C) 1.5 x 10 -5 M<br />
(D) 8.4 x 10 -10 M<br />
4. Silver oxalate Ag 2 (C 2 O 4 ) is dissolved in pure water. The concentration of Ag + ion in a saturated<br />
solution is 2.2 x 10 -4 M. What is the <strong>Ksp</strong> of Ag 2 (C 2 O 4 )?<br />
(A) 5.3 x 10 -12<br />
(B) 1.1 x 10 -12<br />
(C) 5.0 x 10 -8<br />
(D) 2.4 x 10 -8<br />
(E) 2.2 x 10 -4<br />
5. What weight of silver chromate (Ag 2 CrO 4 ) will dissolve in 1.0 liter of a solution that is 0.010 M in Ag + ?<br />
K sp Ag 2 CrO 4 = 1.9 x 10 -12 and FM Ag 2 CrO 4 = 332 g/mol.<br />
(A) 1.9 x 10 -12 g<br />
(B) 1.9 x 10 -8 g<br />
(C) 1.4 x 10 -6 g<br />
(D) 6.3 x 10 -6 g<br />
(E) 4.7 x 10 -4 g<br />
6. The solubility of CaF 2 in 1 x 10 -3 M KF<br />
(A) is equal to the solubility of CaF 2 in pure water.<br />
(B) is less than the solubility of CaF 2 in pure water.<br />
(C) is greater than the solubility of CaF 2 in pure water.<br />
©1994–PTAS, Inc.<br />
Page 1 of 1
<strong>Solubility</strong>, <strong>Ksp</strong> <strong>Worksheet</strong> 1<br />
7. A solution has the following concentrations:<br />
[Cl - ] = 1.5 x 10 -1 M [Br - ] = 5.0 x 10 -4 M [CrO 2- 4 ] = 1.9 x 10 -2 M<br />
A solution of AgNO 3 (100% dissociated) is added to the above solution, drop by drop. Which silver salt<br />
will precipitate first?<br />
K sp AgCl = 1.5 x 10 -10 K sp AgBr = 5.0 x 10 -13 K sp Ag 2 CrO 4 = 1.9 x 10 -12<br />
(A) AgCl<br />
(B) AgBr<br />
(C) Ag 2 CrO 4<br />
(D) AgCl and AgBr together<br />
(E) none of the above<br />
8. Suppose that CaF 2 is to be used as a fluoridation agent in a municipal water system. What is the [F - ] if<br />
extremely hard water ([Ca 2+ ] = 0.070 M) is saturated with CaF 2 ? (K sp CaF 2 = 1.7 x 10 -10 )<br />
(A) 1.3 x 10 -5 M<br />
(B) 2.5 x 10 -5 M<br />
(C) 5.0 x 10 -5 M<br />
(D) 1.0 x 10 -4 M<br />
(E) 0.070 M<br />
9. Which of the following salts is most soluble in water?<br />
(A) Mg(OH) 2 (B) CaS (C) (NH 4 ) 2 CO 3 (D) PbCl 2<br />
10. A 2.00 g sample of a compound requires 268.0 ml of 0.100 M AgNO 3 to precipitate all of the<br />
chloride ion in the compound. What is the percent of Cl - in the compound? (AM of Cl = 35.45)<br />
(A) 47.6% (B) 95.1% (C) 23.8 % (D) 4.75 %<br />
11. Predict what effect each of the following has on the position of the equilibrium.<br />
PbCl 2 Pb 2+ (aq) + 2Cl- (aq)<br />
∆H = +23.4 KJ<br />
(A) The addition of Pb(NO 3 ) 2 solution.<br />
(B) Increase the temperature.<br />
(C) The addition of Ag + .<br />
(D) The addition of HCl.<br />
12. Which salt is the least soluble in water?<br />
(A) Hg 2 CrO 4 (<strong>Ksp</strong> = 2.0 x 10 -9 )<br />
(B) BaF 2 (<strong>Ksp</strong> = 1.7 x 10 -6 )<br />
(C) CaF 2 (<strong>Ksp</strong> = 4.0 x 10 -11 )<br />
(D) AgOH (<strong>Ksp</strong> = 1.5 x 10 -8 )<br />
(E) BaCO 3 (<strong>Ksp</strong> = 8.0 x 10 -9 )<br />
©1994–PTAS, Inc.<br />
Page 2 of 2