Ascension® pyroCarbon Lunate
Ascension® pyroCarbon Lunate
Ascension® pyroCarbon Lunate
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Cyclic<br />
Wear test<br />
Against<br />
Bone<br />
penetration rate<br />
(nanometer/cycle)<br />
0.2<br />
<strong>pyroCarbon</strong><br />
elastic<br />
Modulus<br />
(Gpa)<br />
23<br />
Cortical Bone<br />
4.3<br />
CoCr Alloy<br />
29.4<br />
<strong>pyroCarbon</strong><br />
30.4<br />
Titanium<br />
105<br />
Titanium<br />
34.7<br />
Zirconia<br />
Wear testing shows<br />
that PyroCarbon-on-bone<br />
articulation wear is<br />
significantly less than<br />
that of medical grade<br />
metals and ceramics.<br />
210<br />
Zirconia<br />
The elastic modulus<br />
of PyroCarbon closely<br />
matches that of cortical<br />
bone.<br />
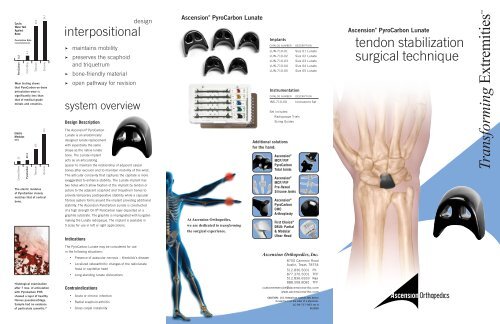
design<br />
interpositional<br />
➤ maintains mobility<br />
➤ preserves the scaphoid<br />
and triquetrum<br />
➤ bone-friendly material<br />
➤ open pathway for revision<br />
system overview<br />
Design Description<br />
The Ascension ® PyroCarbon<br />
<strong>Lunate</strong> is an anatomically<br />
designed lunate replacement<br />
with essentially the same<br />
shape as the native lunate<br />
bone. The <strong>Lunate</strong> implant<br />
acts as an articulating<br />
spacer to maintain the relationship of adjacent carpal<br />
bones after excision and to maintain mobility of the wrist.<br />
The articular concavity that captures the capitate is more<br />
exaggerated to enhance stability. The <strong>Lunate</strong> implant has<br />
two holes which allow fixation of the implant by tendon or<br />
suture to the adjacent scaphoid and triquetrum bones to<br />
provide temporary postoperative stability while a capsular<br />
fibrosis system forms around the implant providing additional<br />
stability. The Ascension PyroCarbon <strong>Lunate</strong> is constructed<br />
of a high strength On-X ® PyroCarbon layer deposited on a<br />
graphite substrate. The graphite is impregnated with tungsten<br />
making the <strong>Lunate</strong> radiopaque. The implant is available in<br />
5 sizes for use in left or right applications.<br />
Indications<br />
Ascension ®<br />
At Ascension Orthopedics,<br />
we are dedicated to transforming<br />
the surgical experience.<br />
PyroCarbon <strong>Lunate</strong><br />
Implants<br />
Catalog number<br />
LUN-710-01<br />
LUN-710-02<br />
LUN-710-03<br />
LUN-710-04<br />
LUN-710-05<br />
Instrumentation<br />
Catalog number<br />
INS-710-00<br />
Set includes:<br />
Radiopaque Trials<br />
Sizing Guides<br />
Additional solutions<br />
for the hand:<br />
Ascension ®<br />
MCP/PIP<br />
PyroCarbon<br />
Total Joints<br />
Ascension ®<br />
MCP/PIP<br />
Pre-flexed<br />
Silicone Joints<br />
Ascension ®<br />
PyroCarbon<br />
CMC<br />
Arthroplasty<br />
First Choice ®<br />
DRUJ: Partial<br />
& Modular<br />
Ulnar Head<br />
description<br />
Size 01 <strong>Lunate</strong><br />
Size 02 <strong>Lunate</strong><br />
Size 03 <strong>Lunate</strong><br />
Size 04 <strong>Lunate</strong><br />
Size 05 <strong>Lunate</strong><br />
description<br />
Instrument Set<br />
Ascension ®<br />
PyroCarbon <strong>Lunate</strong><br />
tendon stabilization<br />
surgical technique<br />
Transforming Extremities <br />
Histological examination<br />
after 7 mos. of articulation<br />
with PyroCarbon PHS<br />
showed a layer of healthy<br />
fibrous pseudocartilage.<br />
Sample had no evidence<br />
of particulate synovitis. 2<br />
The PyroCarbon <strong>Lunate</strong> may be considered for use<br />
in the following situations:<br />
• Presence of avascular necrosis – Kienböck’s disease<br />
• Localized osteoarthritic changes of the radiolunate<br />
fossa or capitellar head<br />
• Long-standing lunate dislocations<br />
Contraindications<br />
• Acute or chronic infection<br />
• Radial scaphoid arthritis<br />
• Gross carpal instability<br />
Ascension Orthopedics, Inc.<br />
8700 Cameron Road<br />
Austin, Texas 78754<br />
512.836.5001 Ph<br />
877.370.5001 TFP<br />
512.836.6933 Fax<br />
888.508.8081 TFF<br />
customerservice@ascensionortho.com<br />
www.ascensionortho.com<br />
Caution: U.S. federal law restricts this device<br />
to sale by or on the order of a physician.<br />
LC-04-717-003 rev A<br />
©2009