Amino Acid Transport
Amino Acid Transport
Amino Acid Transport
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
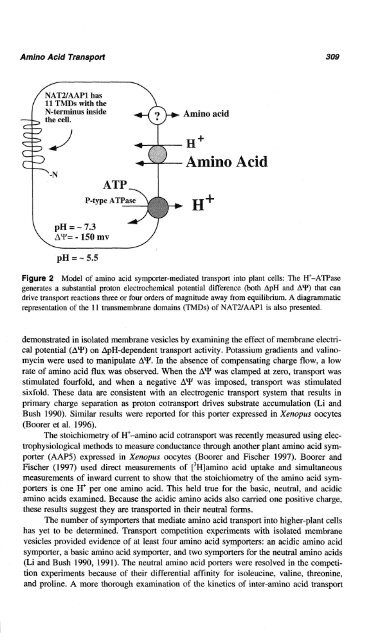
Model of amino acid symporter-mediated transport into plant cells: The H+-ATPase<br />
generates a substantial proton electrochemical potential difference (both ApH and AY) that can<br />
drive transport reactions three or four orders of magnitude away from equilibrium. A diagrammatic<br />
representation of the 11 transmembrane domains (TMDs) of NAT2/AAPl is also presented.<br />
demonstrated in isolated membrane vesicles by examining the effect of membrane electrical<br />
potential (AY) on ApH-dependent transport activity. Potassium gradients and valinomycin<br />
were used to manipulate AY. In the absence of compensating charge flow, a low<br />
rate of amino acid flux was observed. When the AY was clamped at zero, transport was<br />
stimulated fourfold, and when a negative AY was imposed, transport was stimulated<br />
sixfold. These data are consistent with an electrogenic transport system that results in<br />
primary charge separation as proton cotransport drives substrate accumulation (Li and<br />
Bush 1990). Similar results were reported for this porter expressed in Xenopus oocytes<br />
(Boorer et al. 1996).<br />
The stoichiometry of H"-amino acid cotransport was recently measured using electrophysiologic~<br />
methods to measure conductance through another plant amino acid symporter<br />
(AAP5) expressed in Xenopus oocytes (Boorer and Fischer 1997). Boorer and<br />
Fischer (1997) used direct measurements of [3H]amino acid uptake and simultaneous<br />
measurements of inward current to show that the stoich~o~etry of the amino acid symporters<br />
is one w' per one amino acid. This held true for the basic, neutral, and acidic<br />
amino acids examined. Because the acidic amino acids also carried one positive charge,<br />
these results suggest they are transported in their neutral forms.<br />
The number of symporters that mediate amino acid transport into higher-plant cells<br />
has yet to be determined. <strong>Transport</strong> competition experiments with isolated membr~e<br />
vesicles provided evidence of at least four amino acid symporters: an acidic amino acid<br />
symporter, a basic amino acid symporter, and two symporters for the neutral amino acids<br />
(Li and Bush 1990, 1991). The neutral amino acid porters were resolved in the competition<br />
experiments because of their differential affinity for isoleucine, valine, threonine,<br />
and proline. A more thorough examination of the kinetics of inter-amino acid transport