Asphaltene precipitation in crude oils: Theory and experiments
Asphaltene precipitation in crude oils: Theory and experiments
Asphaltene precipitation in crude oils: Theory and experiments
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
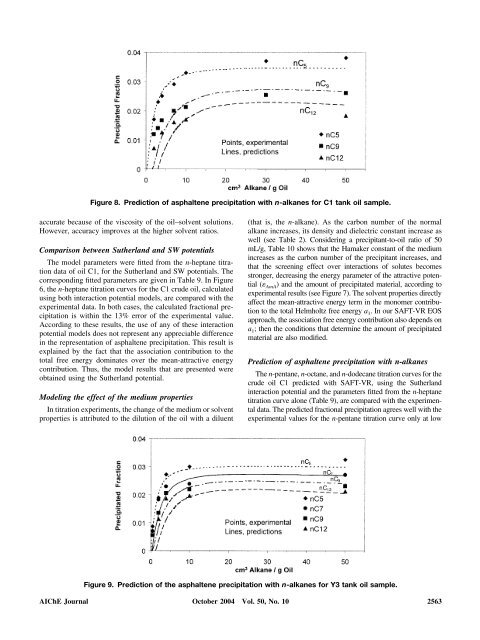
Figure 8. Prediction of asphaltene <strong>precipitation</strong> with n-alkanes for C1 tank oil sample.<br />
accurate because of the viscosity of the oil–solvent solutions.<br />
However, accuracy improves at the higher solvent ratios.<br />
Comparison between Sutherl<strong>and</strong> <strong>and</strong> SW potentials<br />
The model parameters were fitted from the n-heptane titration<br />
data of oil C1, for the Sutherl<strong>and</strong> <strong>and</strong> SW potentials. The<br />
correspond<strong>in</strong>g fitted parameters are given <strong>in</strong> Table 9. In Figure<br />
6, the n-heptane titration curves for the C1 <strong>crude</strong> oil, calculated<br />
us<strong>in</strong>g both <strong>in</strong>teraction potential models, are compared with the<br />
experimental data. In both cases, the calculated fractional <strong>precipitation</strong><br />
is with<strong>in</strong> the 13% error of the experimental value.<br />
Accord<strong>in</strong>g to these results, the use of any of these <strong>in</strong>teraction<br />
potential models does not represent any appreciable difference<br />
<strong>in</strong> the representation of asphaltene <strong>precipitation</strong>. This result is<br />
expla<strong>in</strong>ed by the fact that the association contribution to the<br />
total free energy dom<strong>in</strong>ates over the mean-attractive energy<br />
contribution. Thus, the model results that are presented were<br />
obta<strong>in</strong>ed us<strong>in</strong>g the Sutherl<strong>and</strong> potential.<br />
Model<strong>in</strong>g the effect of the medium properties<br />
In titration <strong>experiments</strong>, the change of the medium or solvent<br />
properties is attributed to the dilution of the oil with a diluent<br />
(that is, the n-alkane). As the carbon number of the normal<br />
alkane <strong>in</strong>creases, its density <strong>and</strong> dielectric constant <strong>in</strong>crease as<br />
well (see Table 2). Consider<strong>in</strong>g a precipitant-to-oil ratio of 50<br />
mL/g, Table 10 shows that the Hamaker constant of the medium<br />
<strong>in</strong>creases as the carbon number of the precipitant <strong>in</strong>creases, <strong>and</strong><br />
that the screen<strong>in</strong>g effect over <strong>in</strong>teractions of solutes becomes<br />
stronger, decreas<strong>in</strong>g the energy parameter of the attractive potential<br />
( AmA ) <strong>and</strong> the amount of precipitated material, accord<strong>in</strong>g to<br />
experimental results (see Figure 7). The solvent properties directly<br />
affect the mean-attractive energy term <strong>in</strong> the monomer contribution<br />
to the total Helmholtz free energy a 1 . In our SAFT-VR EOS<br />
approach, the association free energy contribution also depends on<br />
a 1 ; then the conditions that determ<strong>in</strong>e the amount of precipitated<br />
material are also modified.<br />
Prediction of asphaltene <strong>precipitation</strong> with n-alkanes<br />
The n-pentane, n-octane, <strong>and</strong> n-dodecane titration curves for the<br />
<strong>crude</strong> oil C1 predicted with SAFT-VR, us<strong>in</strong>g the Sutherl<strong>and</strong><br />
<strong>in</strong>teraction potential <strong>and</strong> the parameters fitted from the n-heptane<br />
titration curve alone (Table 9), are compared with the experimental<br />
data. The predicted fractional <strong>precipitation</strong> agrees well with the<br />
experimental values for the n-pentane titration curve only at low<br />
Figure 9. Prediction of the asphaltene <strong>precipitation</strong> with n-alkanes for Y3 tank oil sample.<br />
AIChE Journal October 2004 Vol. 50, No. 10<br />
2563