Controlled hydrogen generation by reaction of aluminum/sodium ...
Controlled hydrogen generation by reaction of aluminum/sodium ...
Controlled hydrogen generation by reaction of aluminum/sodium ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
5812<br />
international journal <strong>of</strong> <strong>hydrogen</strong> energy 37 (2012) 5811e5816<br />
metallic Al using well-established technologies [8,9]. But the<br />
development <strong>of</strong> Al/H 2 O system as practical <strong>hydrogen</strong> source<br />
requires a solution to the long-standing problem <strong>of</strong> surface<br />
passivation <strong>of</strong> Al. Currently, there are a number <strong>of</strong> approaches<br />
under investigation, which can be divided into two categories:<br />
one is addition <strong>of</strong> hydroxides [10,11], metal oxides [12,13] or<br />
selected salts [14,15] to disrupt the passivation layer; the other<br />
is alloying Al with low melting point metals to inhibit the<br />
formation <strong>of</strong> a coherent passivation layer [16e19]. In our study<br />
<strong>of</strong> the Al/H 2 O <strong>reaction</strong> system, we mainly focused on the alkali<br />
approach because the alkali-promoted system always<br />
outperforms other systems in terms <strong>of</strong> HG kinetics. But<br />
meanwhile, the use <strong>of</strong> alkali promoter causes corrosion <strong>of</strong> the<br />
system apparatus. From a practical point <strong>of</strong> view, novel<br />
technologies that enable minimization <strong>of</strong> alkali concentration<br />
without compromising <strong>of</strong> HG performance are highly<br />
desirable.<br />
Quite recently, our study found that a combined usage <strong>of</strong><br />
NaOH and Na 2 SnO 3 can dramatically improve the HG<br />
kinetics <strong>of</strong> the Al/H 2 Osystem[20]. Furthermore, the addition<br />
<strong>of</strong> a small amount <strong>of</strong> Na 2 SnO 3 causes a remarkable decrease<br />
<strong>of</strong> NaOH concentration that is required for achieving favorable<br />
HG performance. This finding provides a simple but<br />
effective method for simultaneously addressing the <strong>reaction</strong><br />
kinetics and alkali corrosion problems <strong>of</strong> the Al/H 2 O system.<br />
Stimulated <strong>by</strong> this finding, we further investigated a new<br />
system, which is composed <strong>of</strong> Al/NaOH/Na 2 SnO 3 solid<br />
mixture and H 2 O. In comparison with the traditional Al/H 2 O<br />
system using aqueous NaOH solution, the new system<br />
exhibits a series <strong>of</strong> advantages on HG performance, manipuility<br />
and adaptability, which makes it promising for<br />
portable <strong>hydrogen</strong> source applications. We herein show the<br />
study results.<br />
2. Experimental<br />
2.1. Preparation <strong>of</strong> solid fuel and sample<br />
characterization<br />
Al powder (99% purity), NaOH (98% purity) and <strong>sodium</strong> stannate<br />
trihydrate (Na 2 SnO 3 $3H 2 O, 99% purity) were purchased<br />
from Sinopharm Chemical Reagent Corporation. In the<br />
present study, a series <strong>of</strong> Al powder samples with different<br />
particle sizes were employed and characterized <strong>by</strong> a Mastersizer<br />
2000 particle size analyzer. Anhydrous <strong>sodium</strong> stannate<br />
(Na 2 SnO 3 ) was prepared <strong>by</strong> calcining Na 2 SnO 3 $3H 2 O at 350 C<br />
for 1 h.<br />
The Al/NaOH/Na 2 SnO 3 powder mixtures in varied mass<br />
ratios were hand-milled using a glass mortar and pestle or<br />
mechanically milled <strong>by</strong> a Fritsch 7 planetary mill at 400 rpm<br />
for 1 h. After the ball-milling, the solid fuel mixture was<br />
pressed into a tablet <strong>by</strong> a pellet machine at 10 MPa for 10 min.<br />
The diameter and height <strong>of</strong> the resulting tablet were 15 and<br />
3 mm, respectively.<br />
The solid residue <strong>of</strong> the Al/H 2 O <strong>reaction</strong> was analyzed <strong>by</strong><br />
powder X-ray diffraction (XRD, Rigaku D/max-2500, Cu Ka<br />
radiation). In preparation <strong>of</strong> the XRD samples, the solid<br />
residue was dried at 80 C under a dynamic vacuum condition<br />
for 48 h.<br />
2.2. Hydrogen <strong>generation</strong> performance testing<br />
The experimental setup used for measurement <strong>of</strong> HG properties<br />
has been described in a previous paper [21]. The <strong>reaction</strong><br />
was carried out in a 250 ml three-neck flask, wherein the solid<br />
fuel was preloaded. The water was fed into contact with the<br />
solid fuel using a pressure-equalizing dropping funnel. Typically,<br />
the feeding rate <strong>of</strong> water was controlled at around<br />
10 g min 1 . The generated <strong>hydrogen</strong> gas passed through<br />
a trap/heat exchanger to cool to room temperature followed<br />
<strong>by</strong> contacting with a silica drier to remove the moisture in the<br />
gas stream. The HG rate was measured using an online mass<br />
flow meter (Sevenstar Huachang, MFM D07e7BM, accuracy<br />
within 2%) that was equipped with a computer. The HG<br />
volume was calculated <strong>by</strong> integrating the measured HG rate<br />
over time. In the whole testing process, no attempt was made<br />
to control the temperature <strong>of</strong> the <strong>reaction</strong> system. The <strong>reaction</strong><br />
temperature was monitored using a thermocouple<br />
embedded in the solid fuel and recorded using an online<br />
recorder. Each experiment was repeated twice. The determined<br />
relative error was no more than 5%.<br />
3. Results and discussion<br />
3.1. Development <strong>of</strong> a new Al-based <strong>hydrogen</strong><br />
<strong>generation</strong> system<br />
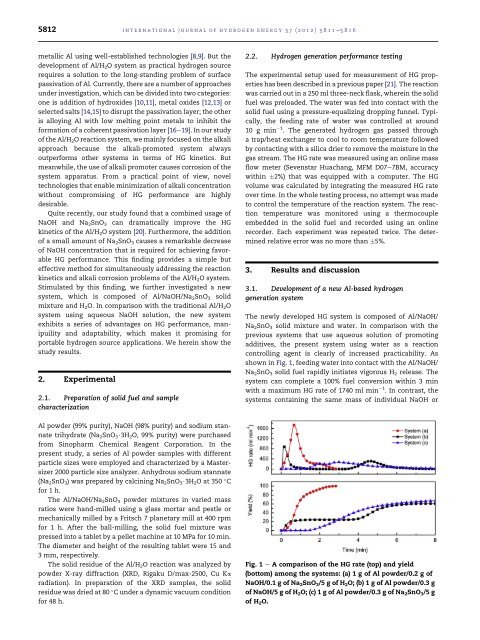
The newly developed HG system is composed <strong>of</strong> Al/NaOH/<br />
Na 2 SnO 3 solid mixture and water. In comparison with the<br />
previous systems that use aqueous solution <strong>of</strong> promoting<br />
additives, the present system using water as a <strong>reaction</strong><br />
controlling agent is clearly <strong>of</strong> increased practicability. As<br />
shown in Fig. 1, feeding water into contact with the Al/NaOH/<br />
Na 2 SnO 3 solid fuel rapidly initiates vigorous H 2 release. The<br />
system can complete a 100% fuel conversion within 3 min<br />
with a maximum HG rate <strong>of</strong> 1740 ml min 1 . In contrast, the<br />
systems containing the same mass <strong>of</strong> individual NaOH or<br />
Fig. 1 e A comparison <strong>of</strong> the HG rate (top) and yield<br />
(bottom) among the systems: (a) 1 g <strong>of</strong> Al powder/0.2 g <strong>of</strong><br />
NaOH/0.1 g <strong>of</strong> Na 2 SnO 3 /5 g <strong>of</strong> H 2 O; (b) 1 g <strong>of</strong> Al powder/0.3 g<br />
<strong>of</strong> NaOH/5 g <strong>of</strong> H 2 O; (c) 1 g <strong>of</strong> Al powder/0.3 g <strong>of</strong> Na 2 SnO 3 /5 g<br />
<strong>of</strong> H 2 O.