Controlled hydrogen generation by reaction of aluminum/sodium ...
Controlled hydrogen generation by reaction of aluminum/sodium ...
Controlled hydrogen generation by reaction of aluminum/sodium ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
5814<br />
international journal <strong>of</strong> <strong>hydrogen</strong> energy 37 (2012) 5811e5816<br />
surface <strong>of</strong> Al may prevent the formation <strong>of</strong> cohesive Al(OH) 3<br />
passivation layer. As a consequence, the Al/H 2 O <strong>reaction</strong> can<br />
proceed in a continuous way, even in the presence <strong>of</strong> low OH<br />
concentration.<br />
3.2. Study <strong>of</strong> <strong>hydrogen</strong> <strong>generation</strong> properties <strong>of</strong> the Al/<br />
NaOH/Na 2 SnO 3 /H 2 O system<br />
As demonstrated, the newly developed Al/NaOH/Na 2 SnO 3 /<br />
H 2 O system possesses a series <strong>of</strong> advantages over the traditional<br />
NaOH-promoted system in terms <strong>of</strong> HG performance.<br />
To evaluate its potential for practical <strong>hydrogen</strong> storage<br />
applications, we further conducted a systematic study <strong>of</strong> the<br />
factors that influence the HG performance <strong>of</strong> the system. The<br />
first factor we examined is the NaOH:Na 2 SnO 3 mass ratio. For<br />
comparison purpose, the quantities <strong>of</strong> Al, H 2 O and promoting<br />
additives were fixed at 1, 5 and 0.3 g, respectively. As seen in<br />
Fig. 4, changing the NaOH:Na 2 SnO 3 mass ratio from 2:1 to 1:2<br />
results in a decrease <strong>of</strong> the HG rate and yield, showing that the<br />
system containing 0.2 g <strong>of</strong> NaOH and 0.1 g <strong>of</strong> Na 2 SnO 3 shows<br />
the best HG performance.<br />
Next, we examined the effect <strong>of</strong> the particle size <strong>of</strong> Al<br />
powder on the HG performance <strong>of</strong> the system. As seen in<br />
Table 1, both the HG rate and yield increase with reducing the<br />
particle size <strong>of</strong> Al powder. This should be ascribed to the<br />
variation <strong>of</strong> the surface area <strong>of</strong> Al. When using Al powder with<br />
an average particle size smaller than 68 mm, the system<br />
exhibited high HG rate and achieved 100% fuel conversion. In<br />
an overall consideration <strong>of</strong> the HG performance, material cost<br />
and availability, we selected the Al powder with an average<br />
particle size <strong>of</strong> 68 mm for further investigation.<br />
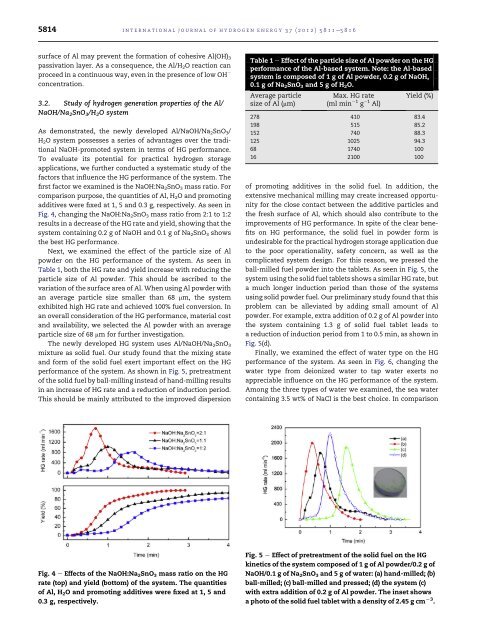
The newly developed HG system uses Al/NaOH/Na 2 SnO 3<br />
mixture as solid fuel. Our study found that the mixing state<br />
and form <strong>of</strong> the solid fuel exert important effect on the HG<br />
performance <strong>of</strong> the system. As shown in Fig. 5, pretreatment<br />
<strong>of</strong> the solid fuel <strong>by</strong> ball-milling instead <strong>of</strong> hand-milling results<br />
in an increase <strong>of</strong> HG rate and a reduction <strong>of</strong> induction period.<br />
This should be mainly attributed to the improved dispersion<br />
Table 1 e Effect <strong>of</strong> the particle size <strong>of</strong> Al powder on the HG<br />
performance <strong>of</strong> the Al-based system. Note: the Al-based<br />
system is composed <strong>of</strong> 1 g <strong>of</strong> Al powder, 0.2 g <strong>of</strong> NaOH,<br />
0.1 g <strong>of</strong> Na 2 SnO 3 and 5 g <strong>of</strong> H 2 O.<br />
Average particle<br />
size <strong>of</strong> Al (mm)<br />
Max. HG rate<br />
(ml min 1 g 1 Al)<br />
Yield (%)<br />
278 410 83.4<br />
198 515 85.2<br />
152 740 88.3<br />
125 1025 94.3<br />
68 1740 100<br />
16 2100 100<br />
<strong>of</strong> promoting additives in the solid fuel. In addition, the<br />
extensive mechanical milling may create increased opportunity<br />
for the close contact between the additive particles and<br />
the fresh surface <strong>of</strong> Al, which should also contribute to the<br />
improvements <strong>of</strong> HG performance. In spite <strong>of</strong> the clear benefits<br />
on HG performance, the solid fuel in powder form is<br />
undesirable for the practical <strong>hydrogen</strong> storage application due<br />
to the poor operationality, safety concern, as well as the<br />
complicated system design. For this reason, we pressed the<br />
ball-milled fuel powder into the tablets. As seen in Fig. 5, the<br />
system using the solid fuel tablets shows a similar HG rate, but<br />
a much longer induction period than those <strong>of</strong> the systems<br />
using solid powder fuel. Our preliminary study found that this<br />
problem can be alleviated <strong>by</strong> adding small amount <strong>of</strong> Al<br />
powder. For example, extra addition <strong>of</strong> 0.2 g <strong>of</strong> Al powder into<br />
the system containing 1.3 g <strong>of</strong> solid fuel tablet leads to<br />
a reduction <strong>of</strong> induction period from 1 to 0.5 min, as shown in<br />
Fig. 5(d).<br />
Finally, we examined the effect <strong>of</strong> water type on the HG<br />
performance <strong>of</strong> the system. As seen in Fig. 6, changing the<br />
water type from deionized water to tap water exerts no<br />
appreciable influence on the HG performance <strong>of</strong> the system.<br />
Among the three types <strong>of</strong> water we examined, the sea water<br />
containing 3.5 wt% <strong>of</strong> NaCl is the best choice. In comparison<br />
Fig. 4 e Effects <strong>of</strong> the NaOH:Na 2 SnO 3 mass ratio on the HG<br />
rate (top) and yield (bottom) <strong>of</strong> the system. The quantities<br />
<strong>of</strong> Al, H 2 O and promoting additives were fixed at 1, 5 and<br />
0.3 g, respectively.<br />
Fig. 5 e Effect <strong>of</strong> pretreatment <strong>of</strong> the solid fuel on the HG<br />
kinetics <strong>of</strong> the system composed <strong>of</strong> 1 g <strong>of</strong> Al powder/0.2 g <strong>of</strong><br />
NaOH/0.1 g <strong>of</strong> Na 2 SnO 3 and 5 g <strong>of</strong> water: (a) hand-milled; (b)<br />
ball-milled; (c) ball-milled and pressed; (d) the system (c)<br />
with extra addition <strong>of</strong> 0.2 g <strong>of</strong> Al powder. The inset shows<br />
a photo <strong>of</strong> the solid fuel tablet with a density <strong>of</strong> 2.45 g cm L3 .