Front Matter - The Journal of Bone & Joint Surgery
Front Matter - The Journal of Bone & Joint Surgery
Front Matter - The Journal of Bone & Joint Surgery
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
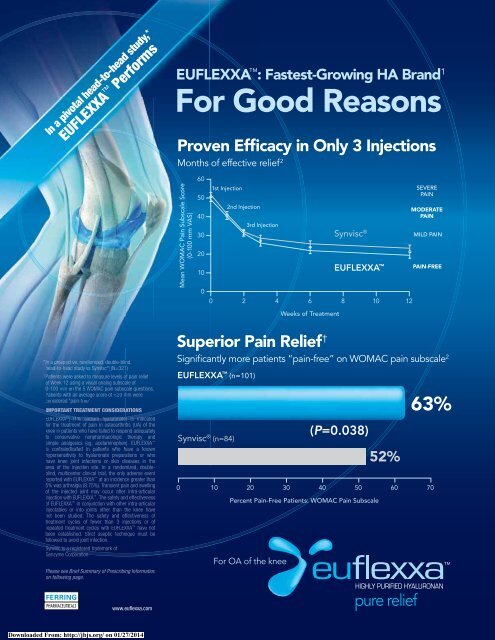
In a pivotal head-to-head study,*<br />
EUFLEXXA Performs<br />
EUFLEXXA : Fastest-Growing HA Brand 1<br />
For Good Reasons<br />
Proven Efficacy in Only 3 Injections<br />
Months <strong>of</strong> effective relief 2<br />
Mean WOMAC Pain Subscale Score<br />
(0-100 mm VAS)<br />
60<br />
50<br />
40<br />
30<br />
20<br />
10<br />
0<br />
1st Injection<br />
2nd Injection<br />
3rd Injection<br />
0 2 4 6 8 10 12<br />
Weeks <strong>of</strong> Treatment<br />
Synvisc ®<br />
EUFLEXXA <br />
SEVERE<br />
PAIN<br />
MODERATE<br />
PAIN<br />
MILD PAIN<br />
PAIN-FREE<br />
* Ina<br />
pr<br />
osp<br />
spect<br />
ective, randomi<br />
zed, double-blind,<br />
head-t dt d-to-head<br />
study vs Synvi<br />
visc<br />
® (N=321)<br />
†<br />
Patients were asked to meas<br />
ure levels <strong>of</strong> pain relief<br />
at Week 12 using a visual analog subscale <strong>of</strong><br />
0-10000 mm on the 5 WOMAC pain subscale questions.<br />
Patients with an averageage<br />
score<br />
<strong>of</strong>
UNIQUE NATIONAL<br />
HCPCS CODE<br />
Q4085<br />
BRIEF SUMMARY<br />
Please consult package insert for full Prescribing Information.<br />
INDICATION<br />
EUFLEXXA (1% sodium hyaluronate) is indicated for the treatment<br />
<strong>of</strong> pain in osteoarthritis (OA) <strong>of</strong> the knee in patients who have failed to<br />
respond adequately to conservative non-pharmacologic therapy and<br />
simple analgesics (e.g., acetaminophen).<br />
CONTRAINDICATIONS<br />
• Do not use EUFLEXXA to treat patients who have a known hypersensitivity<br />
to hyaluronan preparations<br />
• Do not use EUFLEXXA to treat patients with knee joint infections,<br />
infections or skin disease in the area <strong>of</strong> the injection site<br />
WARNINGS<br />
• Mixing <strong>of</strong> quaternary ammonium salts such as benzalkonium chloride with<br />
hyaluronan solutions results in formation <strong>of</strong> a precipitate. EUFLEXXA<br />
should not be administered through a needle previously used with medical<br />
solutions containing benzalkonium chloride. Do not use disinfectants for<br />
skin preparation that contain quaternary ammonium salts<br />
• Do not inject intravascularly because intravascular injection may cause<br />
systemic adverse events<br />
PRECAUTIONS<br />
General<br />
• Patients having repeated exposure to EUFLEXXA have the potential for<br />
an immune response; however, this has not been assessed in humans<br />
• Safety and effectiveness <strong>of</strong> injection in conjunction with other intra-articular<br />
injectables, or into joints other than the knee has not been studied<br />
• Remove any joint effusion before injecting<br />
• Transient pain or swelling <strong>of</strong> the injected joint may occur after intra-articular<br />
injection with EUFLEXXA<br />
• Do not use after expiration date<br />
• Protect from light<br />
• Do not re-use—dispose <strong>of</strong> the syringe after use<br />
• Do not use if the blister package is opened or damaged<br />
Information for Patients<br />
• Transient pain and/or swelling <strong>of</strong> the injected joint may occur after intraarticular<br />
injection <strong>of</strong> EUFLEXXA<br />
• As with any invasive joint procedure, it is recommended that the patient<br />
avoid any strenuous activities or prolonged (i.e., more than 1 hour)<br />
weight-bearing activities such as jogging or tennis within 48 hours<br />
following intra-articular injection<br />
• <strong>The</strong> safety and effectiveness <strong>of</strong> repeated treatment cycles <strong>of</strong> EUFLEXXA<br />
have not been established<br />
ADVERSE EVENTS<br />
Adverse event information regarding the use <strong>of</strong> EUFLEXXA as a treatment<br />
for pain in OA <strong>of</strong> the knee was available from two sources; a multicenter<br />
clinical trial conducted in Germany and a single center clinical trial that was<br />
conducted in Israel.<br />
Multicenter Clinical Investigation<br />
This clinical investigation was a prospective randomized, double blinded,<br />
active control (commercially available hyaluronan product) study conducted<br />
at 10 centers. Three hundred twenty-one patients were randomized into<br />
groups <strong>of</strong> equal size to receive either EUFLEXXA (n=160) or the active<br />
control (n=161). A total <strong>of</strong> 119 patients reported 196 adverse events; this<br />
number represents 54 (33.8%) <strong>of</strong> the EUFLEXXA group and 65 (44.4%) <strong>of</strong><br />
the active control group. <strong>The</strong>re were no deaths reported during the study.<br />
Incidences <strong>of</strong> each event were similar for both groups, except for knee joint<br />
effusion, which was reported by 9 patients in the active control group and<br />
one patient in the EUFLEXXA treatment group. A total <strong>of</strong> 160 patients<br />
received 478 injections <strong>of</strong> EUFLEXXA. <strong>The</strong>re were 27 reported adverse<br />
events considered to be related to EUFLEXXA injections: arthralgia –<br />
11 (6.9%); back pain – 1 (0.63%); blood pressure increase – 3 (1.88%);<br />
joint effusion – 1 (0.63%); joint swelling – 3 (1.88%); nausea – 1 (0.63%);<br />
paresthesia – 2 (1.25%); feeling <strong>of</strong> sickness <strong>of</strong> injection – 3 (1.88%); skin<br />
irritation – 1 (0.63%); tenderness in study knee – 1 (0.63%). Four adverse<br />
events were reported for the EUFLEXXA group that the relationship to<br />
treatment was considered to be unknown: fatigue – 3 (1.88%); nausea –<br />
1 (0.63%).<br />
Single Center Study<br />
In a single-center, single-blinded, placebo controlled, prospective,<br />
two parallel treatment arm clinical trial a total <strong>of</strong> 49 (25 EUFLEXXA,<br />
24 placebo) patients were randomized into two treatment groups in a ratio<br />
<strong>of</strong> 1:1 EUFLEXXA or placebo. A total <strong>of</strong> 65 adverse events were reported<br />
by 17 (68%) <strong>of</strong> the patients in the EUFLEXXA group and 15 (63%) in<br />
the placebo group. Of the 65 total events reported, 20 were regarded as<br />
treatment related. Knee pain, hypokinesia <strong>of</strong> the knee, knee swelling, and<br />
rash were considered to be treatment related adverse events.<br />
DETAILED DEVICE DESCRIPTION<br />
Each syringe <strong>of</strong> EUFLEXXA contains:<br />
Sodium hyaluronate<br />
Sodium chloride<br />
Disodium hydrogen phosphate dodecahydrate<br />
Sodium dihydrogen phosphate dihydrate<br />
Water for injection<br />
20 mg<br />
17 mg<br />
1.12 mg<br />
0.1 mg<br />
q.s.<br />
HOW SUPPLIED<br />
EUFLEXXA is supplied in 2.25 ml nominal volume, disposable, pre-fi lled<br />
glass syringes containing 2 ml <strong>of</strong> EUFLEXXA. Only the contents <strong>of</strong> the syringe<br />
are sterile. EUFLEXXA is nonpyrogenic. 3 disposable syringes per carton.<br />
CAUTION<br />
Product contact parts <strong>of</strong> the syringe contain natural rubber latex,<br />
which may cause allergic reactions.<br />
DIRECTIONS FOR USE<br />
• Store at 2°-25°C (36º-77ºF). Protect from light. Do not freeze.<br />
If refrigerated, remove from refrigeration at least 20-30 minutes<br />
before use.<br />
• EUFLEXXA is administered by intra-articular injection into the knee<br />
synovial capsule using strict aseptic injection procedures. <strong>The</strong> full content<br />
<strong>of</strong> the syringe is injected into the affected knee at weekly intervals for<br />
3 weeks, for a total <strong>of</strong> 3 injections.<br />
• If refrigerated, twenty to thirty minutes before use, remove the product<br />
box from the refrigerator, remove the blister pack from the box and allow<br />
the syringe to come to room temperature. Be sure to return any syringes<br />
not intended for use to the refrigerator.<br />
Toll free number for providers and patients to call with questions:<br />
1-(888)-FERRING (1-(888)-337-7464).<br />
MANUFACTURED FOR:<br />
FERRING PHARMACEUTICALS INC.<br />
PARSIPPANY, NJ 07054<br />
MANUFACTURED BY:<br />
Bio-Technology General (Israel) Ltd.<br />
Be’er Tuvia Industrial Zone, Kiryat Malachi 83104, Israel<br />
Issue date: 05/2006<br />
References: 1. IMS data, March 2007 2. Kirchner M, Marshall D. A double-blind randomized controlled trial<br />
comparing alternate forms <strong>of</strong> high molecular weight hyaluronan for the treatment <strong>of</strong> osteoarthritis <strong>of</strong> the knee.<br />
Osteoarthritis Cartilage. 2006;14:154-162.<br />
©2007 Ferring Pharmaceuticals Inc. 8/07 EUF-75698A<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
Downloaded From: http://jbjs.org/ on 01/27/2014
THE JOURNAL OF BONE & JOINT SURGERY · JBJS.ORG VOLUME 89-A · NUMBER 9 · SEPTEMBER 2007<br />
September 2007<br />
<strong>The</strong> <strong>Journal</strong> <strong>of</strong> <strong>Bone</strong> & <strong>Joint</strong> <strong>Surgery</strong> · American Volume<br />
SCIENTIFIC ARTICLES<br />
1887<br />
Hallux Valgus and First Ray Mobility. Michael J. Coughlin, MD, and Carroll P. Jones, MD<br />
A Prospective Study<br />
1899<br />
Intermediate and Long-Term Outcomes <strong>of</strong><br />
Total Ankle Arthroplasty and Ankle Arthrodesis.<br />
A Systematic Review <strong>of</strong> the Literature<br />
A video supplement to this article has been developed<br />
by the American Academy <strong>of</strong> Orthopaedic Surgeons and JBJS<br />
1906<br />
Removal <strong>of</strong> Painful Orthopaedic<br />
Implants After Fracture Union<br />
1913<br />
Patients’ Preoperative Expectations<br />
Predict the Outcome <strong>of</strong> Rotator Cuff Repair<br />
1920<br />
Minimally Invasive Hip Arthroplasty:<br />
What Role Does Patient<br />
Preconditioning Play?<br />
1928<br />
Clinical and Structural Outcomes<br />
<strong>of</strong> Nonoperative Management<br />
<strong>of</strong> Massive Rotator Cuff Tears<br />
1935<br />
Ultraviolet Lighting During Orthopaedic<br />
<strong>Surgery</strong> and the Rate <strong>of</strong> Infection<br />
1941<br />
<strong>The</strong> Effect <strong>of</strong> Kneeling During Spine<br />
<strong>Surgery</strong> on Leg Intramuscular Pressure<br />
S.L. Haddad, MD, J.C. Coetzee, MD, R. Estok, RN, BSN,<br />
K. Fahrbach, PhD, D. Banel, BA, and L. Nalysnyk, MD, MPH<br />
Reuven B. Minkowitz, MD, Siraj Bhadsavle, MD,<br />
Michael Walsh, PhD, and Kenneth A. Egol, MD<br />
R. Frank Henn III, MD, Robert Z. Tashjian, MD,<br />
Lana Kang, MD, and Andrew Green, MD<br />
Aidin Eslam Pour, MD, Javad Parvizi, MD, FRCS,<br />
Peter F. Sharkey, MD, William J. Hozack, MD, and<br />
Richard H. Rothman, MD, PhD<br />
P.O. Zingg, MD, B. Jost, MD, A. Sukthankar, MD,<br />
M. Buhler, MD, C.W.A. Pfirrmann, MD, and C. Gerber, MD<br />
Merrill A. Ritter, MD, Emily M. Olberding, BS, and<br />
Robert A. Malinzak, MD<br />
Bryan T. Leek, MD, R. Scott Meyer, MD, John M.<br />
Wiemann, MD, Adnan Cutuk, MD, Brandon R.<br />
Macias, BS, and Alan R. Hargens, PhD<br />
How to Reach Us<br />
Editorial and business <strong>of</strong>fices: 20 Pickering Street, Needham, MA 02492-3157. Telephone: (781) 449-9780<br />
www.jbjs.org · e-mail: mail@jbjs.org · Editorial Fax: (781) 449-9787 · Advertising Fax: (781) 449-3485 · Subscription Fax: (781) 449-9742<br />
<strong>The</strong> <strong>Journal</strong> <strong>of</strong> <strong>Bone</strong> & <strong>Joint</strong> <strong>Surgery</strong> (ISSN: 0021-9355) (American Volume) is issued monthly.<br />
<strong>The</strong> 2007 U.S. subscription price, payable in advance, is $162.00. Single copies, $30.00.<br />
<strong>The</strong> <strong>Journal</strong> <strong>of</strong> <strong>Bone</strong> & <strong>Joint</strong> <strong>Surgery</strong>, periodicals postage paid at Boston, Massachusetts, and at additional mailing <strong>of</strong>fices.<br />
Postmaster: Send address changes to <strong>The</strong> <strong>Journal</strong> <strong>of</strong> <strong>Bone</strong> & <strong>Joint</strong> <strong>Surgery</strong>, 20 Pickering Street, Needham, MA 02492-3157.<br />
<strong>The</strong> <strong>Journal</strong> <strong>of</strong> <strong>Bone</strong> and <strong>Joint</strong> <strong>Surgery</strong> ® , JB&JS ® , and JBJS ® are registered in the U.S. Patent and Trademark Office.<br />
COPYRIGHT © 2007 BY THE JOURNAL OF BONE AND JOINT SURGERY, INCORPORATED. ALL RIGHTS RESERVED.<br />
➤<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
EXOGEN ultrasound treatment has the highest<br />
heal rate for non-unions, including patients with<br />
certain comorbidities 1<br />
<strong>The</strong> EXOGEN Ultrasound <strong>Bone</strong> Healing System is like no other:<br />
•Highest heal rate for non-unions – 86% 1<br />
•Accelerates healing <strong>of</strong> indicated* fresh fractures – 38% 2<br />
•Unique fracture healing ultrasound technology<br />
•Works in just 20 minutes a day<br />
1<br />
Highest rate <strong>of</strong> healing reported among pre-market approval submissions to FDA based on variable fracture types, sites and<br />
conditions. See EXOGEN – PMA 900009 – 10/05/1994, EXOGEN – PMA 900009, Supplement – 02/23/2000, Bioelectron – PMA<br />
P850022 – 02/18/1986, EBI – PMA P790002 – 11/06/1979, Orth<strong>of</strong>ix/AME – PMA P850007 – 02/21/1986, Orthologic – PMA P910066<br />
– 03/04/1994, EBI – PMA P790005 – 01/25/1980.<br />
2 Heckman JD, Ryaby JP, McCabe J, Frey JJ, Kilcoyne RF. Acceleration <strong>of</strong> tibial fracture-healing by non-invasive, low-intensity pulsed<br />
ultrasound. J <strong>Bone</strong> <strong>Joint</strong> Surg Am. 1994 Jan;76(1):26–34.<br />
Orthopaedic Trauma & Clinical <strong>The</strong>rapies<br />
Smith & Nephew, Inc. 1450 Brooks Road, Memphis, TN 38116 USA<br />
Telephone: 1-901-396-2121, Information: 1-800-821-5700, Orders and Inquiries: 1-800-836-4080<br />
www.smith-nephew.com www.exogen.com<br />
Summary <strong>of</strong> Indications for Use: <strong>The</strong> EXOGEN 4000+, or any other<br />
EXOGEN <strong>Bone</strong> Healing System, is indicated for the non-invasive<br />
treatment <strong>of</strong> established non-unions † excluding skull and vertebra.<br />
* In addition, they are indicated for accelerating the time to a healed<br />
fracture for fresh, closed, posteriorly displaced distal radius fractures<br />
and fresh, closed or Grade I open tibial diaphysis fractures in skeletally<br />
mature individuals when these fractures are orthopaedically managed<br />
by closed reduction and cast immobilization.<br />
Contraindications: <strong>The</strong>re are no known contraindications for the EXOGEN<br />
device. Warnings and precautions pertaining to the treatment <strong>of</strong> either<br />
condition may be found at www.exogen.com or by calling 1-800-836-4080.<br />
†<br />
A non-union is considered to be established when the fracture site<br />
shows no visibly progressive signs <strong>of</strong> healing. Rx only.<br />
Trademark <strong>of</strong> Smith & Nephew.<br />
Reg. US Pat. & TM Off.<br />
©2007 Smith & Nephew, Inc.<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
THE JOURNAL OF BONE & JOINT SURGERY · JBJS.ORG VOLUME 89-A · NUMBER 9 · SEPTEMBER 2007<br />
September 2007<br />
<strong>The</strong> <strong>Journal</strong> <strong>of</strong> <strong>Bone</strong> & <strong>Joint</strong> <strong>Surgery</strong> · American Volume<br />
SCIENTIFIC ARTICLES<br />
1948<br />
Lateral Unicompartmental Knee Alexander P. Sah, MD, and Richard D. Scott, MD<br />
Arthroplasty Through a Medial Approach.<br />
Study with an Average Five-Year Follow-up<br />
A video supplement to this article will be<br />
available from the Video <strong>Journal</strong> <strong>of</strong> Orthopaedics<br />
1955<br />
Anatomic Factors Related to the<br />
Cause <strong>of</strong> Tennis Elbow<br />
1964<br />
Locking Compression Plate Fixation<br />
<strong>of</strong> Vancouver Type-B1 Periprosthetic<br />
Femoral Fractures<br />
1970<br />
<strong>The</strong> Quality <strong>of</strong> Reporting <strong>of</strong> Orthopaedic<br />
Randomized Trials with Use <strong>of</strong> a<br />
Checklist for Nonpharmacological <strong>The</strong>rapies<br />
1979<br />
Comparison <strong>of</strong> Arthrodesis and<br />
Metallic Hemiarthroplasty <strong>of</strong> the<br />
Hallux Metatarsophalangeal <strong>Joint</strong><br />
1986<br />
Synovectomy <strong>of</strong> the Hip in Patients<br />
with Juvenile Rheumatoid Arthritis<br />
1993<br />
Long-Term Results <strong>of</strong> <strong>Surgery</strong> for<br />
Forearm Deformities in Patients with<br />
Multiple Cartilaginous Exostoses<br />
2000<br />
<strong>The</strong> Anatomy <strong>of</strong> the Medial<br />
Part <strong>of</strong> the Knee<br />
2011<br />
<strong>The</strong> in Vivo Isometric Point <strong>of</strong> the<br />
Lateral Ligament <strong>of</strong> the Elbow<br />
Robert E. Bunata, MD, David S. Brown, MD,<br />
and Roderick Capelo, MD<br />
M.A. Buttaro, MD, G. Farfalli, MD, M. Paredes<br />
Núñez, MD, F. Comba, MD, and F. Piccaluga, MD<br />
Simon Chan, BSc, and Mohit Bhandari, MD<br />
Steven M. Raikin, MD, Jamal Ahmad, MD,<br />
Aidin Eslam Pour, MD, and Nicholas Abidi, MD<br />
Hans-Dieter Carl, Dr. med, Annemarie Schraml, Dr. med,<br />
Bernd Swoboda, Pr<strong>of</strong>. Dr. med, and Gerd Hohenberger, Dr. med<br />
Shosuke Akita, MD, Tsuyoshi Murase, MD, Kazuo<br />
Yonenobu, MD, Kozo Shimada, MD, Kazuhiro Masada, MD,<br />
and Hideki Yoshikawa, MD, PhD<br />
Robert F. LaPrade, MD, PhD, Anders Hauge Engebretsen,<br />
Medical Student, Thuan V. Ly, MD, Steinar Johansen, MD,<br />
Fred A. Wentorf, MS, and Lars Engebretsen, MD, PhD<br />
Hisao Moritomo, MD, PhD, Tsuyoshi Murase, MD, PhD,<br />
Sayuri Arimitsu, MD, Kunihiro Oka, MD, Hideki<br />
Yoshikawa, MD, PhD, and Kazuomi Sugamoto, MD, PhD<br />
➤<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
Downloaded From: http://jbjs.org/ on 01/27/2014
THE JOURNAL OF BONE & JOINT SURGERY · JBJS.ORG VOLUME 89-A · NUMBER 9 · SEPTEMBER 2007<br />
September 2007<br />
<strong>The</strong> <strong>Journal</strong> <strong>of</strong> <strong>Bone</strong> & <strong>Joint</strong> <strong>Surgery</strong> · American Volume<br />
2018<br />
Blood Supply to the First Metatarsal<br />
Head and Vessels at Risk with<br />
a Chevron Osteotomy<br />
SCIENTIFIC ARTICLES<br />
J.J. George Malal, MBBS, DOrtho, MS(Ortho), DNB(Ortho),<br />
MRCS, J. Shaw-Dunn, BSc, MBChB, PhD, FRCS, AIAS,<br />
and C. Senthil Kumar, FRCS(Tr&Orth)<br />
2023<br />
Evaluation <strong>of</strong> Oxidation and Fatigue<br />
Damage <strong>of</strong> Retrieved Crossfire<br />
Polyethylene Acetabular Cups<br />
2030<br />
<strong>The</strong> Location in Cartilage <strong>of</strong><br />
Infectious Retrovirus in Cats Infected<br />
with Feline Leukemia Virus<br />
Barbara H. Currier, MChE, John H. Currier, MS, Michael B. Mayor,<br />
MD, Kimberly A. Lyford, BA, John P. Collier, DE, and Douglas W.<br />
Van Citters, PhD<br />
Steven P. Arnoczky, DVM, Cheryl Swenson, DVM, PhD,<br />
Monika Egerbacher, DVM, PhD, Keri Gardner, MS, Oscar<br />
Caballero, MS, and Meghan Burns, DVM<br />
CASE REPORTS<br />
2037<br />
Epidural Hematoma Causing Paraplegia Joon Y. Lee, MD, Ahmad Nassr, MD, and Ravi K. Ponnappan, MD<br />
After a Fluoroscopically Guided Cervical<br />
Nerve-Root Injection. A Case Report<br />
2040<br />
Surgical Treatment <strong>of</strong> a Tear <strong>of</strong> the<br />
Pectoralis Major Muscle at Its<br />
Sternal Origin. A Case Report<br />
2044<br />
Invasive Group-A Streptococcal<br />
Infection in an Allograft<br />
Recipient. A Case Report<br />
2048<br />
Epidural Hematoma Secondary to<br />
Removal <strong>of</strong> an Epidural Catheter After a<br />
Total Knee Replacement. A Case Report<br />
Michael K. Shindle, MD, Abtin H. Khosravi, MS, Brett M. Cascio,<br />
MD, E. Gene Deune, MD, and Edward G. McFarland, MD<br />
Ellen H. Lee, MD, Dayna Ferguson, MD, Daniel Jernigan, MD, MPH,<br />
Melissa Greenwald, MD, Timothy Coté, MD, Jon E. Bos, MPH,<br />
Jeannette Guarner, MD, Sherif Zaki, MD, PhD, Anne Schuchat, MD,<br />
Bernard Beall, PhD, and Arjun Srinivasan, MD<br />
Sokratis E. Varitimidis, MD, Konstantinos Paterakis, MD,<br />
Zoe H. Dailiana, MD, Michalis Hantes, MD, and Stavroula<br />
Georgopoulou, MD<br />
CURRENT CONCEPTS REVIEW<br />
2051<br />
Management <strong>of</strong> Distal Radial Fractures<br />
Neal C. Chen, MD, and Jesse B. Jupiter, MD<br />
A translation <strong>of</strong> this article is available at jbjs.org.<br />
➤<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
NEW<br />
Designed by<br />
John L. Stanton, MD, FACS<br />
Designed to work as “tissue pushers”, helping to enhance exposure by<br />
allowing the surgeon or an assistant to push forward the opposite side <strong>of</strong><br />
the wound while retracting (or not) the nearer side<br />
Stanton<br />
Forward<br />
Army-Navy<br />
Retractor<br />
Product No’s:<br />
4515-01 [Shallow]<br />
4515-02 [Deep]<br />
Stanton Forward Retractors<br />
Posterior-Inferior<br />
Retractors<br />
Designed for Total Hip <strong>Surgery</strong><br />
Designed by<br />
Wayne M. Goldstein, MD<br />
Stanton Forward<br />
Ragnell Retractor<br />
Product No’s:<br />
4510-01 [Shallow – 13mm]<br />
4510-02 [Deep – 19mm]<br />
<strong>The</strong> posterior-inferior<br />
retractor is placed with<br />
the point at 6 o’clock<br />
and the retractor’s<br />
axilla resting on the<br />
ischium. <strong>The</strong> remaining<br />
blade <strong>of</strong> this retractor<br />
is used to retract the<br />
remaining capsule from<br />
the posterior lip <strong>of</strong> the<br />
acetabulum.<br />
Three<br />
Sizes<br />
Available<br />
Product No’s:<br />
7625-01 [Small Right]<br />
7625-02 [Small Left]<br />
7925-01 [Medium Right]<br />
7925-02 [Medium Left]<br />
7620-01 [Large Right]<br />
7620-02 [Large Left]<br />
Stanton Forward<br />
Senn Retractor<br />
Product No’s:<br />
4520 [Shallow With Teeth]<br />
4525-01 [Shallow – 13mm]<br />
4525-02 [Deep – 19mm]<br />
Sorrells Posterior<br />
Acetabular Retractor<br />
Product No’s:<br />
7320-22A [With Teeth]<br />
Overall Length: 7"<br />
Blade Width: 45mm<br />
7320-22B [Without Teeth]<br />
Overall Length: 7"<br />
Blade Width: 45mm<br />
Designed by<br />
R. Barry Sorrells, MD<br />
NEW STYLE<br />
AVAILABLE<br />
Now available<br />
with and<br />
without teeth<br />
Makes it easier in<br />
certain types <strong>of</strong><br />
surgeries for the<br />
assistant to hold the<br />
wound open without<br />
becoming fatigued and<br />
having to reach around<br />
to the opposite side<br />
<strong>of</strong> the wound to use<br />
another retractor.<br />
Sorrells Anterior/Inferior<br />
Acetabular Retractor<br />
Designed by<br />
R. Barry Sorrells, MD<br />
Product No: 7320-06<br />
FREE TRIAL<br />
ON MOST INSTRUMENTS<br />
CALL FOR MORE INFORMATION<br />
©2007 Innomed, Inc., All Rights Reserved<br />
Phone 800 548 2362<br />
912 236 0000<br />
Fax 912 236 7766<br />
Internet www.innomed.net Innomed<br />
O RTHO PEDIC INS TRUMENTS<br />
eMail info@innomed.net 103 Estus Drive Savannah GA 31404<br />
®<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
THE JOURNAL OF BONE & JOINT SURGERY · JBJS.ORG VOLUME 89-A · NUMBER 9 · SEPTEMBER 2007<br />
September 2007<br />
<strong>The</strong> <strong>Journal</strong> <strong>of</strong> <strong>Bone</strong> & <strong>Joint</strong> <strong>Surgery</strong> · American Volume<br />
SELECTED INSTRUCTIONAL COURSE LECTURE<br />
2064<br />
Operative Carpal and Hand Injuries in Children<br />
Peter M. Waters, MD<br />
THE ORTHOPAEDIC FORUM<br />
2075<br />
AOA Symposium<br />
Gainsharing in Orthopaedics:<br />
Passing Fancy or Wave <strong>of</strong> the Future?<br />
Douglas R. Dirschl, MD, Joane Goodroe, D. McCarty Thornton, and Gary W. Eiland<br />
2084<br />
Follow-up on Misrepresentation <strong>of</strong><br />
Research Activity by Orthopaedic Residency<br />
Applicants: Has Anything Changed?<br />
Emmanuel K. Konstantakos, MD, Richard T. Laughlin, MD,<br />
Ronald J. Markert, PhD, and Lynn A. Crosby, MD<br />
ETHICS IN PRACTICE<br />
2089<br />
Physician Advertising:<br />
Evaluation <strong>of</strong> a Sample Advertisement<br />
James D. Capozzi, MD<br />
SPECIALTY UPDATE<br />
2092<br />
What’s New in Orthopaedic Research<br />
Lawrence V. Gulotta, MD, Chisa Hidaka, MD, Suzanne A. Maher, PhD,<br />
Matthew E. Cunningham, MD, PhD, and Scott A. Rodeo, MD<br />
Follows Table <strong>of</strong> Contents<br />
Instructions to Authors<br />
DEPARTMENTS<br />
2102<br />
Book Reviews<br />
Adv 32, 48, 64, 74, 80, 86, 92, 98, 104, 112, 118<br />
Abstracts from <strong>The</strong> <strong>Journal</strong> <strong>of</strong> <strong>Bone</strong> and <strong>Joint</strong> <strong>Surgery</strong> [Br]<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
<strong>The</strong> marriage <strong>of</strong> the Uni<br />
and the PFJ<br />
A partnership like no other<br />
*smith&nephew<br />
JOURNEY DEUCE<br />
Bi-Compartmental Knee System<br />
• Retention <strong>of</strong> the ACL and PCL for more normal kinematics<br />
• Retention <strong>of</strong> the unaffected lateral compartment<br />
• Replacement <strong>of</strong> the diseased medial and patell<strong>of</strong>emoral compartments –<br />
which could represent up to 70% <strong>of</strong> TKA performed*<br />
Contact your Smith & Nephew sales representative for more information<br />
Orthopaedic Reconstruction<br />
Smith & Nephew, Inc. 1450 Brooks Road, Memphis, TN 38116 USA<br />
Telephone: 901-396-2121, Information: 1-800-821-5700, Orders/Inquiries: 1-800-238-7538<br />
www.smith-nephew.com<br />
* Rolston, L; Sprague, J; Tsai, S; Salehi, A. A Novel <strong>Bone</strong>/Ligament Sparing Prosthesis for the Treatment <strong>of</strong> Patell<strong>of</strong>emoral and Medial Compartment Osteoarthritis. 2006 AAOS Annual Meeting, Poster #P181.<br />
Trademark <strong>of</strong> Smith & Nephew. US Pat. & TM Off.<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
THE JOURNAL OF BONE & JOINT SURGERY · JBJS.ORG VOLUME 89-A · NUMBER 9 · SEPTEMBER 2007<br />
E XCLUSIVELY ON JBJS. ORG!<br />
SEPTEMBER 2007<br />
STREAMING VIDEO OF THE MONTH<br />
September 1-30<br />
VJO/JBJS Featured Streaming Video: “Functional Treatment <strong>of</strong> Fractures,” featuring<br />
Augusto Sarmiento, MD, and Loren L. Latta, PhD (“A Functional Below-the-Knee Brace<br />
for Tibial Fractures: A Report on Its Use in One Hundred Thirty-Five Cases” JBJS,<br />
March 1970, and “A Functional Below-the-Knee Brace for Tibial Fractures: A Report on<br />
Its Use in One Hundred and Thirty-Five Cases,” JBJS September [Suppl 2, Pt 2] 2007)<br />
WEEKLY VIDEO RELEASES FROM THE VIDEO<br />
JOURNAL OF ORTHOPAEDICS AND JBJS<br />
August 28 - October 2<br />
“Chevron Osteotomy for<br />
Correction <strong>of</strong> Hallux<br />
Valgus” featuring Mark S.<br />
Myerson, MD<br />
VIDEO SUPPLEMENTS FROM THE AMERICAN<br />
ACADEMY OF ORTHOPAEDIC SURGEONS AND JBJS<br />
“<strong>The</strong> Agility Total Ankle<br />
Arthroplasty” with Frank<br />
L. Alvine, MD, Steven L.<br />
Haddad, MD, and Charles<br />
L. Saltzman, MD<br />
September 4 - October 9<br />
“Stabilized Subcutaneous<br />
Anterior Ulnar Nerve Transposition”<br />
featuring Steven Z.<br />
Glickel, MD, O. Alton Barron,<br />
MD, and Richard G. Eaton, MD<br />
September 11 - October 16<br />
“Operative Treatment <strong>of</strong> DDH<br />
with Open Reduction and Salter<br />
Osteotomy” featuring Stuart L.<br />
Weinstein, MD, and Dennis R.<br />
Wenger, MD<br />
September 18 - October 23<br />
“Elbow Arthroscopy: Avoiding<br />
Complications” featuring<br />
Bernard F. Morrey, MD<br />
“Distraction Plating <strong>of</strong> Distal<br />
Radial Fractures with Metaphyseal<br />
and Diaphyseal<br />
Comminution” with David<br />
S. Ruch, MD<br />
“Cementation <strong>of</strong> Constrained<br />
Liner into Secure Cementless<br />
Acetabular Shells: A Two to<br />
Twelve-Year Follow-Up Study”<br />
with John Callaghan, MD<br />
“<strong>The</strong> Vastus-Splitting Approach<br />
for Primary Total<br />
Knee Arthroplasty” with<br />
Vincent D. Pellegrini Jr., MD<br />
September 25 - October 30<br />
“Ankle Arthrodesis for Posttraumatic<br />
Arthritis” featuring<br />
Charles L. Saltzman, MD<br />
“Arthroscopic Débridement<br />
<strong>of</strong> Acetabular Labral Tears”<br />
with John C. Clohisy, MD<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
Downloaded From: http://jbjs.org/ on 01/27/2014
THE JOURNAL OF BONE & JOINT SURGERY · JBJS.ORG VOLUME 89-A · NUMBER 9 · SEPTEMBER 2007<br />
ON JBJS.ORG (CONTINUED)<br />
COMMENTARY AND PERSPECTIVE<br />
• Mark Easley, MD, on “Hallux Valgus and First Ray<br />
Mobility. A Prospective Study,” by Coughlin and Jones<br />
(JBJS, September 2007)<br />
• Thomas J. Gill, MD, on “Patients’ Preoperative<br />
Expectations Predict the Outcome <strong>of</strong> Rotator Cuff<br />
Repair,” by Henn et al. (JBJS, September 2007)<br />
• Mark W. Pagnano, MD, on “Minimally Invasive<br />
Hip Arthroplasty: What Role Does Patient Preconditioning<br />
Play?” by Pour et al. (JBJS, September 2007)<br />
ONLINE CME ANNOUNCEMENT<br />
JBJS is pleased to announce that our online quarterly<br />
CME examination has been approved by the ABOS as<br />
a Self-Assessment Examination (SAE).<br />
Earn 10 <strong>of</strong> your 20 required SAE credits with the<br />
successful completion <strong>of</strong> the JBJS online quarterly<br />
general CME exam.<br />
IMAGE QUIZ<br />
• Suprapubic Pain Following<br />
Strenuous Physical Activity<br />
IMAGE-QUIZ LIBRARY<br />
• A Cervical Spine Injury<br />
Secondary to a Motor-Vehicle<br />
Accident<br />
• Enlarging Painless Mass in a Four-Year-Old Girl<br />
• Painless Shoulder Mass in a Fifty-Six-Year-Old<br />
Woman<br />
• An Unusual High-Energy Injury to the Ankle<br />
• Pathologic Fracture <strong>of</strong> the Humerus During<br />
Pregnancy<br />
• Hip Pain in a Seventeen-Year-Old Girl<br />
• Destructive Fibular Lesion in a Ten-Year-Old Boy<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
INSTRUCTIONS TO AUTHORS<br />
THE JOURNAL OF BONE & JOINT SURGERY · JBJS.ORG VOLUME 89-A · NUMBER 9 · SEPTEMBER 2007<br />
Instructions to Authors<br />
<strong>The</strong> <strong>Journal</strong> <strong>of</strong> <strong>Bone</strong> and <strong>Joint</strong> <strong>Surgery</strong> welcomes articles that contribute to orthopaedic<br />
knowledge from all sources in all countries. • Articles are accepted only for exclusive<br />
publication in <strong>The</strong> <strong>Journal</strong> <strong>of</strong> <strong>Bone</strong> and <strong>Joint</strong> <strong>Surgery</strong>. Previously published articles, even<br />
those in peer-reviewed electronic publications, are not accepted by <strong>The</strong> <strong>Journal</strong>. • Publication<br />
does not constitute <strong>of</strong>ficial endorsement <strong>of</strong> opinions presented in articles. • Published<br />
articles and illustrations become the property <strong>of</strong> <strong>The</strong> <strong>Journal</strong>. • If the Editor-in-<br />
Chief <strong>of</strong> <strong>The</strong> <strong>Journal</strong> requests additional data forming the basis for the work, the authors<br />
will make the data available for examination in a timely fashion. • All manuscripts dealing<br />
with the study <strong>of</strong> human subjects must include a statement that the subjects gave<br />
Informed Consent to participate in the study and that the study has been approved by<br />
an institutional review board or a similar committee. All Case Reports must include a<br />
statement that each subject was informed that data concerning the case would be submitted<br />
for publication. All studies should be carried out in accordance with the World<br />
Medical Association Declaration <strong>of</strong> Helsinki, as presented in <strong>The</strong> <strong>Journal</strong> (1997;79-A:<br />
1089-98). Patient confidentiality must be protected according to the U.S. Health Insurance<br />
Portability and Accountability Act (HIPAA). • All clinical trials submitted for consideration<br />
should have been registered in a public trials registry. • All manuscripts dealing<br />
with experimental results in animals must include a statement that the study has been<br />
approved by an animal utilization study committee. <strong>The</strong> authors should also include<br />
information about the management <strong>of</strong> postoperative pain for both animal and human<br />
subjects. • Reports <strong>of</strong> randomized controlled trials (RCTs) should follow the checklist<br />
developed by the CONSORT Group (www.consort-statement.org), published in JAMA<br />
2001;285:1987-91. In the preparation <strong>of</strong> a manuscript, authors should, in general, follow<br />
the recommendations in “Uniform Requirements for Manuscripts Submitted to Biomedical<br />
<strong>Journal</strong>s: Writing and Editing for Biomedical Publication” by the International<br />
Committee <strong>of</strong> Medical <strong>Journal</strong> Editors, October 2004 (www.icmje.org). • On occasion,<br />
reviewers, associate editors, and/or deputy editors may have a conflict <strong>of</strong> interest or a<br />
competing interest with regard to the subject matter <strong>of</strong> a manuscript. Such conflicts are<br />
disclosed to the Editor-in-Chief, who has no known conflicts <strong>of</strong> interest or competing<br />
interests and who makes the final decision regarding acceptance or rejection <strong>of</strong> all<br />
manuscripts submitted to <strong>The</strong> <strong>Journal</strong>.<br />
Submission <strong>of</strong> Manuscript<br />
<strong>The</strong> <strong>Journal</strong> <strong>of</strong> <strong>Bone</strong> and <strong>Joint</strong> <strong>Surgery</strong> uses a<br />
web-based service, provided by Editorial Manager,<br />
requiring authors to submit and track<br />
manuscripts electronically. Authors must register<br />
via the Internet address jbjs.edmgr.com.<br />
You will be e-mailed a confidential username<br />
and password that will enable you to access the<br />
system and submit your manuscript.<br />
When you submit an article, the following<br />
items must be included:<br />
1. Title Page: List the title <strong>of</strong> the manuscript<br />
and the authors’ names in the order in<br />
which they should appear. Provide a<br />
complete mailing address for each author.<br />
Clearly designate the corresponding author<br />
and his/her mailing address, telephone<br />
number, fax number, and e-mail address.<br />
2. Blinded Manuscript: <strong>The</strong> <strong>Journal</strong> <strong>of</strong> <strong>Bone</strong><br />
and <strong>Joint</strong> <strong>Surgery</strong> has a policy <strong>of</strong> blinded<br />
peer review. <strong>The</strong> manuscript must not<br />
contain any mention <strong>of</strong> the authors’<br />
names or initials or the institution at<br />
which the study was done. Page headers<br />
can include the title but not the authors’<br />
names. Manuscripts not in compliance<br />
with <strong>The</strong> <strong>Journal</strong>’s blinding policy will be<br />
returned to the corresponding author.<br />
3. IRB Approval: A copy <strong>of</strong> the letter granting<br />
approval from the institutional review<br />
board or the animal utilization<br />
study committee is required. You must<br />
reference the manuscript title and corresponding<br />
author on the fax cover sheet or<br />
in an accompanying letter.<br />
4. Copyright Transfer and Author Agreement:<br />
Material appearing in <strong>The</strong> <strong>Journal</strong> is<br />
covered by copyright. All authors must sign<br />
a Copyright Transfer and Author Agreement<br />
form upon submission <strong>of</strong> the manuscript<br />
to <strong>The</strong> <strong>Journal</strong>. <strong>The</strong> form must reference<br />
the manuscript title, assigned JBJS<br />
manuscript number, and corresponding<br />
author. This form must be submitted by<br />
post mail, by fax, or in PDF format online.<br />
5. Potential Conflict <strong>of</strong> Interest Statement:<br />
Authors <strong>of</strong> manuscripts must sign<br />
a Conflict <strong>of</strong> Interest Statement at the time<br />
<strong>of</strong> submission <strong>of</strong> each manuscript. <strong>The</strong><br />
form must reference the manuscript title<br />
and assigned JBJS manuscript number.<br />
This statement has no bearing on the editorial<br />
decision to publish a manuscript.<br />
That decision will continue to be based<br />
solely on the value <strong>of</strong> the article to the<br />
readers <strong>of</strong> <strong>The</strong> <strong>Journal</strong>. <strong>The</strong> signature <strong>of</strong><br />
each author is required. No article will be<br />
published until the completed conflict <strong>of</strong><br />
interest form has been incorporated into<br />
the record kept on that manuscript in <strong>The</strong><br />
<strong>Journal</strong> <strong>of</strong>fice. <strong>The</strong> statements selected by<br />
the author or authors will be printed at the<br />
end <strong>of</strong> the published article.<br />
Forms required for manuscript submission<br />
can be found at jbjs.org.<br />
When you submit an article, the following<br />
items are optional:<br />
1. Cover Letter<br />
2. Acknowledgment: If included, it must be<br />
attached as a separate file, not included in<br />
the text <strong>of</strong> the manuscript.<br />
3. Figures and/or Tables: Figures must be<br />
submitted electronically. Each figure<br />
and/or table must be labeled separately<br />
and submitted as a separate electronic<br />
file. No more than 30 figures may be<br />
submitted. Tables should be submitted<br />
in their original file format. Refer to the<br />
section entitled Illustrations for figure<br />
format requirements.<br />
<strong>The</strong> <strong>Journal</strong> discourages submission <strong>of</strong> illustrations<br />
that have been published elsewhere.<br />
When such illustrations are deemed<br />
essential, the author must include a letter,<br />
from the original holder <strong>of</strong> the copyright,<br />
granting permission to reprint the illustration.<br />
Give full information about the previous<br />
publication, including the page on<br />
which the illustration appeared.<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
INSTRUCTIONS TO AUTHORS<br />
THE JOURNAL OF BONE & JOINT SURGERY · JBJS.ORG VOLUME 89-A · NUMBER 9 · SEPTEMBER 2007<br />
Preparation <strong>of</strong> Manuscript<br />
Manuscripts should be a maximum <strong>of</strong> 5000<br />
words. <strong>The</strong>y must be double-spaced with<br />
wide margins. Pages must be numbered<br />
sequentially. An article should consist <strong>of</strong>:<br />
1. A structured abstract <strong>of</strong> no more than<br />
325 words, consisting <strong>of</strong> five paragraphs,<br />
with the headings Background (which<br />
states the primary research question),<br />
Methods, Results, Conclusions, and Level <strong>of</strong><br />
Evidence (for clinical articles) or Clinical<br />
Relevance (for basic-science articles). For<br />
the Level <strong>of</strong> Evidence section, describe the<br />
study type and assign a level-<strong>of</strong>-evidence<br />
rating to the primary research question,<br />
according to the criteria in the table in the<br />
Instructions to Authors. Do not include an<br />
abstract with case reports.<br />
2. <strong>The</strong> body should consist <strong>of</strong>:<br />
Introduction: State the problem that<br />
led to the study, including a concise<br />
review <strong>of</strong> only the relevant literature.<br />
State your hypothesis and the purpose<br />
<strong>of</strong> the study.<br />
Materials and Methods: Describe<br />
the study design (prospective or retrospective,<br />
inclusion and exclusion criteria,<br />
duration <strong>of</strong> study) and the study<br />
population (demographics, duration<br />
<strong>of</strong> follow-up).<br />
Statistical Methods should be described<br />
in detail. Use <strong>of</strong> the word significant<br />
requires reporting <strong>of</strong> a p value.<br />
Ninety-five percent confidence intervals<br />
are required whenever the results <strong>of</strong><br />
survivorship analysis are given in the<br />
text or graphs. Use <strong>of</strong> the word correlation<br />
requires reporting <strong>of</strong> the correlation<br />
coefficient.<br />
<strong>The</strong> <strong>Journal</strong> encourages the use <strong>of</strong> validated<br />
outcome instruments. <strong>The</strong> use <strong>of</strong><br />
both a generic (general) health outcome<br />
measure and a joint-specific, limb-specific,<br />
or condition-specific instrument is<br />
encouraged. If an outcome system leads<br />
to a categorical ranking (excellent, good,<br />
etc.), the aggregate score for each patient<br />
should be provided.<br />
Results: Provide a detailed report on<br />
the data obtained during the study. Results<br />
obtained after less than two years <strong>of</strong><br />
follow-up are rarely accepted. All data in<br />
the text must be consistent throughout<br />
the manuscript, including any illustrations,<br />
legends, or tables.<br />
Discussion: Be succinct. What does your<br />
study show? Is your hypothesis affirmed or<br />
refuted? Discuss the importance <strong>of</strong> this article<br />
with regard to the relevant world literature;<br />
a complete literature review is<br />
unnecessary. Analyze your data and discuss<br />
their strengths, their weaknesses, and<br />
the limitations <strong>of</strong> the study.<br />
3. Illustrations accompanying your manuscript<br />
must be submitted electronically<br />
and be in TIFF or EPS format. Do not<br />
import images into other s<strong>of</strong>tware programs.<br />
No more than 30 images may be<br />
submitted.<br />
Any digital manipulation <strong>of</strong> an image—<br />
color, contrast, brightness, etc.—must be<br />
applied to the entire image and may not<br />
result in misrepresentation <strong>of</strong> the original<br />
image. Enhancement or alteration <strong>of</strong> part<br />
<strong>of</strong> an image, without clear and explicit disclosure<br />
in the legend, is unacceptable.<br />
Image files should be named appropriately<br />
and include the number <strong>of</strong> the figure (e.g.,<br />
Figure1.tif, Figure2.eps, etc.). When completing<br />
the online submission form, remember<br />
to enter the name and number<br />
<strong>of</strong> the figure (Figure 1, Figure 2, etc.) into<br />
the “description” field. This description<br />
should match the name <strong>of</strong> the image file.<br />
Color images must be RGB (not CMYK).<br />
We cannot alter or vouch for the quality<br />
<strong>of</strong> color reproductions.<br />
In accordance with HIPAA, remove any<br />
writing that could identify the patient<br />
(e.g., names, initials, patient numbers).<br />
When using a digital camera to create<br />
your images, if possible, set the camera<br />
to save in TIFF format (not JPEG), set the<br />
resolution to a minimum <strong>of</strong> 300 ppi (pixels<br />
per inch), and set the size <strong>of</strong> the image<br />
to 5 × 7 in (127 × 178 mm).<br />
<strong>The</strong> resolution <strong>of</strong> your electronic images<br />
is critical and is directly linked to<br />
how well they will appear when printed.<br />
Color and grayscale images, such as<br />
radiographs, must have a minimum<br />
resolution <strong>of</strong> 300 ppi, and line-art drawings<br />
must have a minimum resolution<br />
<strong>of</strong> 1200 ppi. An original image size <strong>of</strong><br />
5 × 7 in (127 × 178 mm) is preferred.<br />
For questions regarding electronic submission<br />
<strong>of</strong> images, contact the Desktop<br />
Publishing Department at dtp@jbjs.org.<br />
4. Legends must be included for all illustrations<br />
and listed in order. Explain what<br />
each illustration shows. Give the magnification<br />
<strong>of</strong> all photomicrographs. Define all arrows<br />
and other such indicators appearing<br />
on the illustration. If an illustration is <strong>of</strong> a<br />
patient who is identified by a case number,<br />
include that case number in the legend.<br />
How to contact us:<br />
<strong>The</strong> <strong>Journal</strong> <strong>of</strong> <strong>Bone</strong> and <strong>Joint</strong> <strong>Surgery</strong><br />
20 Pickering Street, Needham, Massachusetts 02492-3157<br />
telephone: 781.449.9780 • fax: 781.449.9787 • e-mail: mail@jbjs.org<br />
5. A bibliography, <strong>of</strong> references made in the<br />
text. Abstracts or meeting transactions<br />
more than three years old should not be<br />
cited. <strong>The</strong> references should be numbered<br />
according to the order <strong>of</strong> citation in the<br />
text (not alphabetically) and should be in<br />
PubMed/Index Medicus format (for an example,<br />
go to the National Center for Biotechnology<br />
Information [NCBI] web site<br />
www.ncbi.nlm.nih. gov/entrez/query.fcgi and<br />
search for specific reference citations). All<br />
references must be cited in the text.<br />
Style<br />
Use “Uniform Requirements for Manuscripts<br />
Submitted to Biomedical <strong>Journal</strong>s:<br />
Writing and Editing for Biomedical Publication”<br />
by the International Committee<br />
<strong>of</strong> Medical <strong>Journal</strong> Editors, October 2004<br />
(www.icmje.org) for standard format. For<br />
style guidelines, use “Scientific Style and<br />
Format. <strong>The</strong> CBE Manual for Authors, Editors,<br />
and Publishers, 6th ed.,” published by<br />
Cambridge University Press.<br />
<strong>The</strong> following style conventions should be<br />
kept in mind:<br />
1. <strong>The</strong> numerator and denominator should<br />
be included for all percentages. Round <strong>of</strong>f<br />
percentages when the denominator is less<br />
than 200. Percentages should not be used<br />
when the value <strong>of</strong> n is less than twenty.<br />
2. All measurements should be given in metric<br />
or SI units, which are abbreviated.<br />
3. No other abbreviations or acronyms<br />
should be used.<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
INSTRUCTIONS TO AUTHORS<br />
THE JOURNAL OF BONE & JOINT SURGERY · JBJS.ORG VOLUME 89-A · NUMBER 9 · SEPTEMBER 2007<br />
Authorship<br />
<strong>The</strong> order <strong>of</strong> names reflects only the preference<br />
<strong>of</strong> the authors. Each author must have<br />
contributed significantly to one or more<br />
aspects <strong>of</strong> the study: its design, data acquisition,<br />
analysis and interpretation <strong>of</strong> data,<br />
drafting <strong>of</strong> the manuscript, critical revision<br />
<strong>of</strong> the manuscript, statistical analysis, and/<br />
or study supervision. Each author should<br />
be able to defend and assume full responsibility<br />
for the content <strong>of</strong> the manuscript, regardless<br />
<strong>of</strong> the specific contributions. No<br />
more than six authors should be listed; individuals<br />
who have contributed to only one<br />
segment <strong>of</strong> the manuscript or have contributed<br />
only cases should be credited in an acknowledgment<br />
footnote. If there are more<br />
than six authors, an accompanying letter <strong>of</strong><br />
transmittal must detail why the authors<br />
have taken exception to these recommendations<br />
and should state how each author<br />
has contributed to the manuscript, with<br />
use <strong>of</strong> the criteria listed above.<br />
If a research group is designated as the<br />
author <strong>of</strong> an article, one or more group<br />
members who fully meet the above criteria<br />
for authorship should be listed in the article’s<br />
byline, followed by “on behalf <strong>of</strong> the<br />
[name <strong>of</strong> group].” <strong>The</strong> other group members<br />
should be listed in an acknowledgment<br />
section at the end <strong>of</strong> the article.<br />
Alternatively, the byline can include only<br />
the name <strong>of</strong> the group, followed by an asterisk<br />
corresponding to a list that specifies<br />
the authors who fully meet the above criteria<br />
for authorship and that also mentions<br />
the other group members.<br />
Letters to <strong>The</strong> Editor<br />
<strong>The</strong> <strong>Journal</strong> welcomes readers’ comments on<br />
published articles. Letters will be accepted and<br />
edited at the Editor’s discretion and will be<br />
published electronically on jbjs.org. Selected<br />
letters and author responses will be published<br />
in the print journal on a quarterly basis. Instructions<br />
for submitting a Letter to the Editor<br />
are available on our website (click “Instructions<br />
to Authors” and then click “Instructions<br />
for submitting a Letter to the Editor”).<br />
Review <strong>of</strong> Manuscripts<br />
Manuscripts are evaluated by the editorial<br />
staff <strong>of</strong> <strong>The</strong> <strong>Journal</strong> and are sent to outside<br />
reviewers. <strong>The</strong> time between receipt <strong>of</strong> a<br />
submitted manuscript and the decision<br />
regarding its publication has averaged six<br />
weeks, but it can be longer.<br />
Levels <strong>of</strong> Evidence for Primary Research Question 1<br />
<strong>The</strong>rapeutic Studies⎯<br />
Investigating the<br />
Results <strong>of</strong> Treatment<br />
Level I • High-quality randomized controlled trial<br />
with statistically significant difference<br />
or no statistically significant difference<br />
but narrow confidence intervals<br />
• Systematic review 2 <strong>of</strong> Level-I randomized<br />
controlled trials (and study results<br />
were homogeneous 3 )<br />
Level II • Lesser-quality randomized controlled<br />
trial (e.g.,
INSTRUCTIONS TO AUTHORS<br />
THE JOURNAL OF BONE & JOINT SURGERY · JBJS.ORG VOLUME 89-A · NUMBER 9 · SEPTEMBER 2007<br />
A CONCISE FORMAT FOR REPORTING THE<br />
LONGER-TERM FOLLOW-UP STATUS OF<br />
PATIENTS MANAGED WITH TOTAL HIP<br />
ARTHROPLASTY<br />
This format is to be used when the original fulllength<br />
article was published in <strong>The</strong> <strong>Journal</strong> <strong>of</strong><br />
<strong>Bone</strong> and <strong>Joint</strong> <strong>Surgery</strong>.<br />
Length limit: Six manuscript pages, excluding<br />
references and figures.<br />
Follow-up intervals: No less than five years since<br />
the previous publication and preferably at five or<br />
ten-year intervals, as long as no interim changes<br />
have occurred that require expedited reporting.<br />
Abstract<br />
State, in a maximum <strong>of</strong> 150 words, why you are<br />
reporting the results at this interval and your<br />
major findings.<br />
Background<br />
Briefly summarize and cite the original study<br />
published in <strong>The</strong> <strong>Journal</strong> <strong>of</strong> <strong>Bone</strong> and <strong>Joint</strong> <strong>Surgery</strong>.<br />
Describe the original:<br />
• patient cohort<br />
• type <strong>of</strong> arthroplasty and critical aspects <strong>of</strong> surgical<br />
and cementing or cementless techniques<br />
• type <strong>of</strong> series (Was this a selected or unselected<br />
series? A consecutive series? Were<br />
the operations performed by a single surgeon?<br />
By multiple surgeons? At multiple institutions?<br />
Were data acquired prospectively<br />
or retrospectively?)<br />
Methods<br />
List, but do not describe, the methods used to<br />
assess clinical and radiographic results and<br />
cite the appropriate reference.<br />
For reporting clinical results:<br />
• you may use the same assessment scheme<br />
employed in your previous report—e.g., Harris,<br />
Hospital for Special <strong>Surgery</strong>, Iowa, Mayo Clinic,<br />
or Merle d’Aubigné-Postel rating system<br />
• you are strongly encouraged to include the<br />
WOMAC scores for the current cohort<br />
• you are encouraged to use the clinical and<br />
radiographic nomenclature described by<br />
Johnston et al. (J <strong>Bone</strong> <strong>Joint</strong> Surg Am.<br />
1990;72:161-8) for other pertinent data<br />
• you must perform survivorship analyses (with<br />
calculation <strong>of</strong> confidence limits) using end<br />
points that are appropriate to your cohort<br />
Results<br />
<strong>The</strong> results should include:<br />
• the original number <strong>of</strong> patients/hips studied<br />
and the number <strong>of</strong> patients/hips studied<br />
since the last report<br />
• the number <strong>of</strong> patients/hips who died, the<br />
number <strong>of</strong> patients/hips who were lost to<br />
follow-up, and the number <strong>of</strong> patients/hips<br />
currently being studied<br />
• the number <strong>of</strong> patients/hips in the updated<br />
series who were examined, the number who<br />
responded to questionnaires, and the number<br />
with available radiographs<br />
• the number <strong>of</strong> patients/hips in whom the primary<br />
joint replacement is still intact<br />
• basic demographic characteristics <strong>of</strong> the cohort,<br />
especially any that might affect results<br />
(age, diagnosis, gender, height, weight, and<br />
level <strong>of</strong> activity)<br />
• the number <strong>of</strong> arthroplasties revised for any<br />
reason. If the revised arthroplasties are included<br />
in the current series, report the status<br />
in this group separately<br />
• complications since the last report, including<br />
infection, dislocation, stem breakage, osteolysis,<br />
wear, and so on<br />
For survivorship analysis, the following end<br />
points should be used:<br />
(1) revision for any cause—e.g., aseptic loosening,<br />
osteolysis, component breakage, or<br />
infection<br />
(2) revision for aseptic loosening <strong>of</strong> the femoral<br />
component<br />
(3) revision for aseptic loosening <strong>of</strong> the acetabular<br />
component<br />
(4) definite radiographic loosening <strong>of</strong> the femoral<br />
component, according to the criteria <strong>of</strong><br />
Harris et al. (J <strong>Bone</strong> <strong>Joint</strong> Surg Am.<br />
1982;64:1063-7) for cemented stems and<br />
the criteria <strong>of</strong> Engh et al. (J <strong>Bone</strong> <strong>Joint</strong> Surg<br />
Br. 1987;69:45-55) for uncemented stems.<br />
If your results cannot be evaluated with<br />
these criteria, cite the appropriate reference<br />
for your rating criteria<br />
(5) definite radiographic loosening <strong>of</strong> the acetabular<br />
component, according to the criteria<br />
<strong>of</strong> Hodgkinson et al. (Clin Orthop.<br />
1988;228:105-9)—i.e., migration or >1<br />
mm <strong>of</strong> radiolucency in all DeLee and<br />
Charnley zones. If your results cannot be<br />
evaluated with these criteria, cite the appropriate<br />
reference for your rating criteria<br />
Conclusions<br />
<strong>The</strong> conclusions should include:<br />
• major factors limiting the longevity <strong>of</strong> the<br />
prosthesis at the time <strong>of</strong> this follow-up<br />
• recommendations regarding the continued<br />
use <strong>of</strong> the prosthesis if it is still available<br />
• if the prosthesis is not still available, lessons<br />
applicable to the current successor or<br />
to similar designs<br />
A CONCISE FORMAT FOR REPORTING THE<br />
LONGER-TERM FOLLOW-UP STATUS OF<br />
PATIENTS MANAGED WITH TOTAL KNEE<br />
ARTHROPLASTY<br />
This format is to be used when the original fulllength<br />
article was published in <strong>The</strong> <strong>Journal</strong> <strong>of</strong><br />
<strong>Bone</strong> and <strong>Joint</strong> <strong>Surgery</strong>.<br />
Length limit: Six manuscript pages, excluding<br />
references and figures.<br />
Follow-up intervals: No less than five years since<br />
the previous publication and preferably at five or<br />
ten-year intervals, as long as no interim changes<br />
have occurred that require expedited reporting.<br />
Abstract<br />
State, in a maximum <strong>of</strong> 150 words, why you are<br />
reporting the results at this interval and your<br />
major findings.<br />
Background<br />
Briefly summarize and cite the original study<br />
published in <strong>The</strong> <strong>Journal</strong> <strong>of</strong> <strong>Bone</strong> and <strong>Joint</strong> <strong>Surgery</strong>.<br />
Describe the original:<br />
• patient cohort<br />
• type <strong>of</strong> arthroplasty and critical aspects<br />
<strong>of</strong> surgical and cementing or cementless<br />
techniques<br />
• type <strong>of</strong> series (Was this a selected or unselected<br />
series? A consecutive series? Were<br />
the operations performed by a single surgeon?<br />
By multiple surgeons? At multiple<br />
institutions? Were data acquired prospectively<br />
or retrospectively?)<br />
Methods<br />
List, but do not describe, the methods used to<br />
assess clinical and radiographic results and<br />
cite the appropriate reference.<br />
For reporting clinical results:<br />
• you may use the same assessment scheme<br />
employed in your previous report—e.g., Hospital<br />
for Special <strong>Surgery</strong> or Knee Society rating<br />
system<br />
• you are strongly encouraged to include the<br />
WOMAC scores for the current cohort<br />
• you are encouraged to use the clinical and<br />
radiographic nomenclature described by<br />
Insall et al. (Clin Orthop. 1989;248:13-4) and<br />
Ewald (Clin Orthop. 1989;248:9-12) for other<br />
pertinent data<br />
• you must perform survivorship analyses (with<br />
calculation <strong>of</strong> confidence limits) using end<br />
points that are appropriate to your cohort<br />
Results<br />
<strong>The</strong> results should include:<br />
• the original number <strong>of</strong> patients/knees studied<br />
and the number <strong>of</strong> patients/knees studied<br />
since the last report<br />
• the number <strong>of</strong> patients/knees who died, the<br />
number <strong>of</strong> patients/knees who were lost to<br />
follow-up, and the number <strong>of</strong> patients/knees<br />
currently being studied<br />
• the number <strong>of</strong> patients/knees in the updated<br />
series who were examined, the number who<br />
responded to questionnaires, and the number<br />
with available radiographs<br />
• the number <strong>of</strong> patients/knees in whom the<br />
primary joint replacement is still intact<br />
• basic demographic characteristics <strong>of</strong> the cohort,<br />
especially any that might affect results<br />
(age, diagnosis, gender, height, weight, and<br />
level <strong>of</strong> activity)<br />
• the number <strong>of</strong> arthroplasties revised for any<br />
reason. If the revised arthroplasties are included<br />
in the current series, report the status<br />
in this group separately<br />
• complications since the last report, including<br />
infection, loosening, component breakage,<br />
osteolysis, wear, instability, and so on<br />
For survivorship analysis, the following end<br />
points should be used:<br />
(1) revision for any cause—e.g., aseptic loosening,<br />
osteolysis, component breakage, instability,<br />
or infection<br />
(2) revision for aseptic loosening <strong>of</strong> the femoral<br />
component<br />
(3) revision for aseptic loosening <strong>of</strong> the tibial<br />
component<br />
(4) revision for aseptic loosening <strong>of</strong> the patellar<br />
component<br />
Conclusions<br />
<strong>The</strong> conclusions should include:<br />
• major factors limiting the longevity <strong>of</strong> the<br />
prosthesis at the time <strong>of</strong> this follow-up<br />
• recommendations regarding the continued<br />
use <strong>of</strong> the prosthesis if it is still available<br />
• if the prosthesis is not still available, lessons<br />
applicable to the current successor or<br />
to similar designs<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
AOA ~ KELLOGG<br />
LEADERSHIP SERIES<br />
~ EXPANDING YOUR STRENGTHS ~<br />
MODULE 5: THE POWER OF INFORMATION<br />
IS IN THE NUMBERS<br />
SEPTEMBER 14-16, 2007<br />
NEW!<br />
“It was as enjoyable as it was enlightening to learn about management from such outstanding authorities.”<br />
Richard H. Gelberman, MD, Washington University School <strong>of</strong> Medicine<br />
(Modules 1 & 2 Attendee)<br />
Master the Indispensible Management Skills that you did not learn in medical school in<br />
a venue designed to specifically meet the needs <strong>of</strong> orthopaedic surgeons. Module 5 will:<br />
• Reveal What the Numbers Really Mean<br />
• Provide Essential Tools to Help Doctors Better Forecast Resource Needs<br />
• Explore Fundraising Tactics & Techniques<br />
• Assess Financial Performance with Essential Tools & Indicators<br />
REGISTER FOR MODULE 5 TODAY!<br />
WWW.AOASSN.ORG OR CALL 847.318.7330.<br />
THE ORDER IS UP TO YOU! <strong>The</strong> modules are designed to be<br />
taken in any sequence. Each module <strong>of</strong>fers interactive,<br />
engaging courses and world-renowned instructors<br />
that discuss issues related to the theme <strong>of</strong> the module.<br />
1<br />
3 6<br />
5<br />
2<br />
4<br />
Module 5 is exclusively supported by an unrestricted educational grant from DePuy, a Johnson & Johnson Company<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
A. B.<br />
We specialize in Plan B.<br />
Zimmer Trauma. Tools to succeed when bone quality fails.<br />
Surgeons around the world count on Zimmer for the most advanced implant<br />
systems. With a complete Trauma portfolio, they can have equal confidence in<br />
Zimmer solutions for periprosthetic fractures. Due to the rising prevalence <strong>of</strong><br />
osteoporosis, the need for flexible intraoperative solutions is growing. <strong>The</strong> NCB ®<br />
Locking Plate system uses standard techniques and delivers the ability to confidently<br />
target cortical and cancellous screws with polyaxial freedom and to lock<br />
the screws at any time during the procedure. In addition, Zimmer ® Periarticular<br />
Plating Systems feature low-pr<strong>of</strong>ile, precontoured plates that require little or no<br />
additional bending. We also have a complete portfolio <strong>of</strong> nailing solutions, hip<br />
fixation and bone void fillers. When a “plan B” is required to manage periprosthetic<br />
fractures, count on the company you already trust. To learn more, contact<br />
your Zimmer representative or visit us at www.zimmer.com.<br />
www.zimmer.com<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
PUBLISHED IN TWO VOLUMES<br />
AMERICAN VOLUME<br />
Editor and Chairman, Board <strong>of</strong> Editors: JAMES D. HECKMAN<br />
Editors Emeriti: PAUL H. CURTISS JR.,<br />
Deputy Editors Emeriti for Research: ALBERT H. BURSTEIN,<br />
HENRY R. COWELL LAWRENCE C. ROSENBERG<br />
ROBERT W. BUCHOLZ, Deputy Editor for Adult Reconstructive<br />
<strong>Surgery</strong> and Trauma<br />
CHARLES R. CLARK, Deputy Editor for Adult Reconstruction and Spine<br />
THOMAS A. EINHORN, Deputy Editor for Current Concepts Reviews<br />
VERNON T. TOLO, Deputy Editor for Pediatric Orthopaedics<br />
VINCENT D. PELLEGRINI Jr., Deputy Editor for Surgical Techniques<br />
ROBERT POSS, Deputy Editor for Electronic Media<br />
DEMPSEY S. SPRINGFIELD, Deputy Editor for Instructional Course Lectures<br />
PAUL TORNETTA III, Deputy Editor for AOA Publications<br />
american editorial board<br />
PETER STERN, Deputy Editor for the Upper Extremity<br />
KEN YAMAGUCHI, Deputy Editor for the Upper Extremity<br />
MARC F. SWIONTKOWSKI, Deputy Editor for Outcome Studies and Trauma<br />
BERTRAM ZARINS, Consulting Editor for Sports Medicine<br />
JAMES G. WRIGHT, Associate Editor for Evidence-Based Orthopaedics<br />
JAMES D. CAPOZZI, Coordinator <strong>of</strong> Ethics in Practice<br />
BRENT GRAHAM, Deputy Editor for Methodology and Biostatistics<br />
JEFFREY N. KATZ, Deputy Editor for Methodology and Biostatistics<br />
ELENA LOSINA, Deputy Editor for Methodology and Biostatistics<br />
JOHN G. BIRCH, Dallas, Texas<br />
R. DALE BLASIER, Little Rock, Arkansas<br />
MARTIN I. BOYER, St. Louis, Missouri<br />
EDWARD Y. CHENG, Minneapolis, Minnesota<br />
SCOTT S. KELLEY, Durham, North Carolina<br />
SETH S. LEOPOLD, Seattle, Washington<br />
BASSAM A. MASRI, Vancouver, British Columbia, Canada<br />
JAMES P. MCAULEY, London, Ontario, Canada<br />
ROBERT M. SZABO, Sacramento, California<br />
EDWARD M. WOJTYS, Ann Arbor, Michigan<br />
the journal <strong>of</strong> bone and<br />
joint surgery incorporated<br />
Board <strong>of</strong> Trustees<br />
MICHAEL A. SIMON, Chairman<br />
BERNARD A. RINEBERG, Vice-Chairman<br />
JAMES H. HERNDON, Treasurer<br />
CECIL H. RORABECK, Secretary<br />
JAMES D. HECKMAN, Editor<br />
DAN M. SPENGLER<br />
VERNON T. TOLO<br />
EDWARD N. HANLEY Jr.<br />
STUART L. WEINSTEIN<br />
the american orthopaedic association<br />
President: PETER STERN<br />
6300 North River Road<br />
Rosemont, Illinois 60018<br />
the american academy <strong>of</strong><br />
orthopaedic surgeons<br />
President: JAMES H. BEATY<br />
6300 North River Road<br />
Rosemont, Illinois 60018<br />
ANTHONY CATTERALL, Chairman<br />
ROBERT DICKSON, Treasurer<br />
NEIL THOMAS, Secretary<br />
JAMES SCOTT, Editor<br />
FRANK HORAN, Editor Emeritus<br />
RICHARD VILLAR, Assistant Editor<br />
ANDREW J. CARR, Associate Editor<br />
JOHN FIXSEN, Associate Editor<br />
ANDREW JACKSON, Associate Editor<br />
DAVID JONES, Associate Editor<br />
DAVID L. LIMB, Associate Editor for Education<br />
ANDREW W. McCASKIE, Associate Editor<br />
ALISTAIR ROSS, Associate Editor<br />
GARETH SCOTT, Associate Editor<br />
british council <strong>of</strong> management<br />
ANTHONY CATTERALL, Chairman<br />
ROBERT DICKSON, Treasurer<br />
NEIL THOMAS, Secretary<br />
ROBERT MARSHALL<br />
JOHN GETTY, President, B.O.A. ex <strong>of</strong>ficio<br />
british orthopaedic association<br />
JOHN GETTY, President<br />
At the Royal College <strong>of</strong> Surgeons<br />
35-43 Lincoln’s Inn Fields, London WC2A 3PN<br />
australian orthopaedic association<br />
JOHN HARRIS, President<br />
Ground Floor, William Bland Centre,<br />
229 Macquarie Street,<br />
Sydney, New South Wales 2000<br />
canadian orthopaedic association<br />
MARC J. MOREAU, President<br />
4150 Ste. Catherine Street West<br />
Suite 360, Westmount, Quebec H3Z 2Y5<br />
board <strong>of</strong> consulting editors<br />
for research<br />
THOMAS W. BAUER, Deputy Editor for Research<br />
CLARE M. RIMNAC, Deputy Editor for Research<br />
H. CLARKE ANDERSON, Kansas City, Kansas<br />
STEVEN ARNOCZKY, East Lansing, Michigan<br />
SCOTT BODEN, Atlanta, Georgia<br />
THOMAS D. BROWN, Iowa City, Iowa<br />
JOSEPH A. BUCKWALTER, Iowa City, Iowa<br />
CHARLES CORNELL, New York, N.Y.<br />
KATHLEEN DERWIN, Cleveland, Ohio<br />
MICHAEL KLEIN, Birmingham, Alabama<br />
KEITH MARKOLF, Los Angeles, California<br />
EDWARD McCARTHY Jr., Baltimore, Maryland<br />
HARRY A. McKELLOP, Los Angeles, California<br />
JAVAD PARVIZI, Philadelphia, Pennsylvania<br />
TERRANCE D. PEABODY, Chicago, Illinois<br />
SCOTT A. RODEO, New York, N.Y.<br />
BRITISH VOLUME<br />
Editor: JAMES SCOTT<br />
Editor Emeritus: FRANK HORAN<br />
british editorial board<br />
NEIL RUSHTON, Deputy Editor for Research Emeritus<br />
LESLIE KLENERMAN, Associate Editor Emeritus<br />
MICHAEL LAURENCE, Associate Editor Emeritus<br />
JOHN GETTY, President, B.O.A., ex <strong>of</strong>ficio<br />
NICOLA MAFFULLI, Editorial Secretary, B.O.A.<br />
GEORGE BENTLEY, London, England (EFORT)<br />
CHARLES COURT-BROWN, Edinburgh, Scotland<br />
CY FRANK, Calgary, Canada<br />
PETER GIANNOUDIS, Leeds, England<br />
S. GOVENDER, Congella, South Africa<br />
ROBERT GRIMER, Birmingham, England<br />
ALEXANDER HADJIPAVLOU, Crete, Greece<br />
JOE W.M. HARPER, Leicester, England<br />
ROBERT HAWKINS, Vancouver, Canada<br />
new zealand orthopaedic association<br />
MURRAY FOSBENDER, President<br />
P.O. Box 7451, Wellington South, New Zealand<br />
south african orthopaedic association<br />
D.F. du POEN LOUW, President<br />
P.O. Box 25846, Langehovenpark 9330, Bloemfontein<br />
irish orthopaedic association<br />
FRANK DOWLING, President<br />
Cappagh National Orthopaedic Hospital, Finglas, Dublin 11<br />
european federation <strong>of</strong><br />
national associations <strong>of</strong> orthopaedics<br />
and traumatology - efort<br />
WOLFHART PUHL, President<br />
Technoparkstrasse 1, CH-8005 Zürich, Switzerland<br />
western orthopaedic association<br />
President: GERARD L. GLANCY<br />
154-A West Foothill Boulevard, #107<br />
Upland, California 91786<br />
eastern orthopaedic association<br />
President: SCOTT D. BODEN<br />
110 West Road, Suite 227<br />
Towson, Maryland 21204<br />
mid-america<br />
orthopaedic association<br />
President: TIMOTHY C. FITZGIBBONS<br />
20 Second Avenue, S.W.<br />
Rochester, Minnesota 55902<br />
DAVID HUNT, London, England<br />
IVAN HVID, Aarhus, Denmark (E.O.R.S.)<br />
GREGORY JANES, Perth, Australia<br />
ROGER LEMAIRE, Liège, Belgium<br />
ROBERT MARSHALL, Reading, England<br />
RÉMY NIZARD, Paris, France<br />
MARK PATERSON, London, England<br />
ANDREW ROBINSON, Cambridge, England<br />
BRIGITTE SCAMMELL (B.O.R.S.)<br />
GEORGE SIKORSKI, Perth, Australia<br />
SUSAN STOTT, Auckland, New Zealand<br />
DAVID WARWICK, Southampton, England<br />
ANDREW WILLIAMS, London, England<br />
JOHAN WITT, London, England<br />
british orthopaedic research society<br />
B. CATERSON, President<br />
c/o BOA<br />
35-43 Lincoln’s Inn Fields, London WC2A 3PN<br />
european orthopaedic research society<br />
NICO VERDONSCHOT, President<br />
Weiner Medizinische Akademie für ärztliche Fortbildung und Forshung,<br />
Vienna Academy <strong>of</strong> Postgraduate Medical Education and Research,<br />
Alserstrasse 4, 1. H<strong>of</strong>, DION, A-1090 Wien<br />
canadian orthopaedic research society<br />
JAMES A. JOHNSON, President<br />
4150 Ste. Catherine Street West<br />
Suite 360, Westmount, Quebec H3Z 2Y5<br />
British Office<br />
22 Buckingham Street, London WC2N 6ET, England<br />
General Manager: STEPHEN BISHOP<br />
Editorial Production Manager: EMMA VODDEN<br />
Circulation Manager: MICHAEL SEARLE<br />
Published in the United Kingdom by<br />
<strong>The</strong> British Editorial Society <strong>of</strong> <strong>Bone</strong> & <strong>Joint</strong> <strong>Surgery</strong><br />
Downloaded From: http://jbjs.org/ on 01/27/2014
Downloaded From: http://jbjs.org/ on 01/27/2014
EDITORIAL STAFF<br />
AMERICAN OFFICE<br />
20 Pickering Street, Needham, Massachusetts 02492-3157 U.S.A.<br />
Editor: JAMES D. HECKMAN, MD<br />
General Manager: MADY TISSENBAUM<br />
Managing Editor: AMY BORDIUK<br />
Subscription Manager: ABRAHAM N. BRODY<br />
Advertising Director: AMBER HOWARD<br />
Marketing Director: MICHELLE HACHÉ<br />
Controller: GEORGE S. HILL<br />
Published and printed in the United States by <strong>The</strong> <strong>Journal</strong> <strong>of</strong> <strong>Bone</strong> and <strong>Joint</strong> <strong>Surgery</strong>, Incorporated<br />
INFORMATION FOR SUBSCRIBERS<br />
Subscription Manager, ABRAHAM N. BRODY<br />
Assistant Subscription Manager, MERLE ADELSON<br />
To start or stop a subscription, to change your<br />
address, or for non-receipt <strong>of</strong> issues: ext. 1241<br />
Fax: 781.449.9742<br />
e-mail: subs@jbjs.org<br />
JBJS-A is a benefit <strong>of</strong> AAOS Active Fellowship.<br />
2007 SUBSCRIPTION RATES<br />
american volume: $162.00 in usa; $176.30 outside usa<br />
published monthly<br />
american and british volumes: $268.00, combined rate<br />
british volume: $132.00<br />
published monthly<br />
Abstracts from the British Volume appear<br />
in this issue and are listed in the Table <strong>of</strong> Contents<br />
Resident/fellow discount rates and complimentary subscriptions<br />
are available, contact subs@jbjs.org for details.<br />
subscription dvd or cd-rom (quarterly)<br />
combined volume:<br />
Residents/fellows, $172.00; JBJS subscribers, $215.00;<br />
non-subscribers, $268.00<br />
Above subscription rates apply to individuals only.<br />
For institutional subscription rates,<br />
please contact the subscription department.<br />
ONLINE INFORMATION<br />
Online access to the full text plus additional electronic features<br />
is included in the print subscription price. Visit us at:<br />
www.jbjs.org<br />
www.jbjs.org.uk<br />
INFORMATION FOR AUTHORS<br />
For questions concerning<br />
submission <strong>of</strong> manuscripts or status<br />
<strong>of</strong> submitted manuscripts: ext. 1210<br />
Fax: 781.449.9787<br />
e-mail: editorial@jbjs.org<br />
Managing Editor, AMY BORDIUK<br />
Editorial Manager, ALLAN HARPER<br />
For questions regarding manuscript style and format: ext. 1243<br />
e-mail: copyediting@jbjs.org<br />
ADVERTISING<br />
Director, AMBER HOWARD<br />
e-mail: howarda@jbjs.org, ext. 1233<br />
Production Manager, BEVERLY OUANNASS,<br />
e-mail: ouannass@jbjs.org, ext. 1234<br />
Classified/Recruitment Coordinator, TERRY PAOLINO,<br />
e-mail: paolinot@jbjs.org, ext. 1239<br />
Advertising Sales Executive, SUSAN O’HAGAN<br />
e-mail: ohagans@jbjs.org, ext. 1229<br />
Advertising Department Fax: 781.449.3485<br />
Pharmaceutical Advertising Representative, JAMES T. BRADY, INC.<br />
Phone: 516.742.7960 Fax: 516.742.7908<br />
e-mail: jtbrady@webspan.net<br />
INFORMATION ON VIDEO SUPPLEMENTS<br />
Video <strong>Journal</strong> <strong>of</strong> Orthopaedics<br />
P.O. Box 4367, Santa Barbara, CA 93140<br />
Phone: 805.962.3410<br />
Fax: 805.962.1260<br />
e-mail: customersupport@vjortho.com<br />
url: www.vjortho.com<br />
JBJS CHANGE OF ADDRESS FORM (NOTIFY US 60 DAYS IN ADVANCE OF CHANGE)<br />
Old Address (Fill in Old Address Here)<br />
Name _____________________________________________<br />
Address____________________________________________<br />
City __________________ State ____ Zip Code _________<br />
New Address (Fill in New Address Here)<br />
Name _____________________________________________<br />
Address ____________________________________________<br />
City __________________ State_____ Zip Code _________<br />
How to Reach Us<br />
Editorial and Business <strong>of</strong>fices: 20 Pickering Street, Needham, Massachusetts 02492-3157. Telephone: 781.449.9780<br />
web site: jbjs.org · e-mail: mail@jbjs.org · Editorial Fax: 781.449.9787 · Advertising Fax: 781.449.3485 · Subscription Fax: 781.449.9742<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
PREP SCHOOL<br />
LESSON NO. 2<br />
COVERAGE<br />
AREA<br />
Some surgical prep applicators<br />
cover a lot more ground<br />
than others.<br />
How much does it cost to cover 450 sq. in.?<br />
With 3M DuraPrep Surgical Solution (Iodine<br />
Povacrylex [0.7% available iodine] and Isopropyl<br />
1<br />
26 mL applicator<br />
<strong>of</strong> DuraPrep<br />
Solution<br />
x<br />
$ _____________<br />
(per applicator cost)<br />
=<br />
$ _____________<br />
2.58<br />
26 mL applicators<br />
<strong>of</strong> ChloraPrep<br />
Antiseptic Prep<br />
x<br />
$ _____________<br />
(per applicator cost)<br />
=<br />
$ _____________<br />
Alcohol, 74% w/w) Patient Preoperative Skin<br />
Preparation, you can cover at least a 15” x 30” area<br />
(450 sq. in.) with a single 8630 applicator. That’s<br />
more than twice the area (13.2” x 13.2”, or 174 sq. in.)<br />
<strong>of</strong> the large 26mL ChloraPrep ® Antiseptic Skin Prep<br />
applicator. Want the facts? Ask your 3M representative<br />
for a Prep School Lesson Book or call the 3M Health Care<br />
Helpline at 1-800-228-3957.<br />
© 3M 2007. All Rights Reserved. DuraPrep and 3M are trademarks <strong>of</strong> 3M. ChloraPrep is a registered trademark <strong>of</strong> Enturia.<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
For MIS and Navigation Assisted<br />
Hip Arthroplasty<br />
US Patent No. 7,100,225<br />
MorphBoard ® Positioning System<br />
• Multiple peg lengths for greater versatility and<br />
better stability<br />
• Lightweight, modular components make set up and<br />
storage easy<br />
• Modular system converts from standard to bariatric<br />
patients by a simple turn <strong>of</strong> the center board component<br />
• Disposable pads and sleeves protect patient from<br />
cross contamination<br />
Universal Lateral Positioner <br />
• Independently adjustable and removable<br />
anterior arms for more accurate contact with<br />
iliac crests<br />
• Patented Hyperflexion Plate allows up to<br />
120º <strong>of</strong> flexion during ROM testing<br />
• Removable Inferior arm to accommodate<br />
any patient anatomy<br />
• Storage case, w/casters for easy handling,<br />
keeps components organized and accessible<br />
US Patent No. 6,003,176<br />
Also from IMP…<br />
De Mayo Hip Positioner <br />
• Simple, adjustable lateral decubitus positioning<br />
• Easy nursing setup for large and obese patients<br />
SlimLine Abduction Pillow<br />
• Assures greater patient comfort and easier<br />
nursing care after hip surgery<br />
Contact your local IMP<br />
representative or call 800-467-4944<br />
IMP... THE OPERATIVE WORD IN<br />
PATIENT POSITIONING<br />
IMP and MorphBoard are registered trademarks, and Universal<br />
Lateral Positioner, De Mayo Hip Positioner and Slimline are<br />
trademarks <strong>of</strong> Innovative Medical Products, Inc.<br />
7173 ©2007 Innovative Medical Products, Inc.<br />
All rights reserved. 8/07<br />
Innovative<br />
Medical Products, Inc.<br />
AGLOBAL LEADER IN PATIENT POSITIONING<br />
87 Spring Lane, Plainville, CT 06062<br />
PH: 860-793-0391 FAX: 860-793-8975<br />
Toll Free: PH: 800-467-4944 FAX: 888-229-1452<br />
www.impmedical.com<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
Stepping forward with locking technology.<br />
Acumed has taken locking technology<br />
one step further with the forefoot<br />
and midfoot plating system. Engineered<br />
with a new counterbore/countersink<br />
design, the locking screws “disappear”<br />
down into the plate. By adding this<br />
technology to the anatomically<br />
contoured, low pr<strong>of</strong>ile plates,<br />
Acumed has provided yet another<br />
innovative solution that’s truly<br />
steps ahead <strong>of</strong> the rest.<br />
(888) 627-9957 www.acumed.net<br />
Downloaded From: http://jbjs.org/ on 01/27/2014<br />
AcUMEDr
Downloaded From: http://jbjs.org/ on 01/27/2014
Could you be treating<br />
your total knee<br />
candidates with an<br />
Oxford ® Partial Knee?<br />
• Less postoperative pain 1 *<br />
• Less bone removal 1 *<br />
• More natural motion 1 *<br />
• Clinically proven at 10, 15 and 20 years 2<br />
Oxford ® Partial Knee<br />
Contact your local Biomet Distributor for more<br />
information about the most widely used partial<br />
knee in the world.<br />
* Compared to total knee replacement<br />
1. Kim, K.T. et al. A Prospective Analysis <strong>of</strong> Oxford Phase 3 Unicompartmental<br />
Knee Arthroplasty. Orthopedics. 30(5 Suppl): 15–18, 2007.<br />
2. Price, A.J. et al. 20-year survival and 10-year clinical results <strong>of</strong> the<br />
Oxford uni knee arthroplasty. Paper No. 046. 73rd Annual AAOS<br />
Meeting. Chicago, IL. 2006.<br />
Biologics • Bracing • Micr<strong>of</strong>i xation • Orthopedics • Osteobiologics • Spine • Sports Medicine • Trauma • 3i<br />
biomet.com • 800.348.9500 x 1501<br />
©2007 Biomet Orthopedics, Inc. Oxford ® is a registered trademark <strong>of</strong> Biomet Manufacturing Corp.<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
Downloaded From: http://jbjs.org/ on 01/27/2014
<strong>The</strong> Science <strong>of</strong> Increased Motion 13<br />
High flexion knee system designed for mobility<br />
with stability through 150 degrees <strong>of</strong> motion.<br />
Flared Posterior Condyles<br />
• Open design accommodates 20 degrees<br />
<strong>of</strong> internal/external rotation throughout<br />
range <strong>of</strong> motion. 7<br />
Rotary Arc Insert<br />
• Precision-machined surface facilitates<br />
internal/external rotation.<br />
Anatomic Radius (10º – 110º)<br />
• Designed to maintain collateral<br />
ligament stability throughout the range<br />
<strong>of</strong> motion. 8<br />
• Centered at the transepicondylar axis -<br />
the optimal flexion axis <strong>of</strong> the knee. 9<br />
Anatomic Patell<strong>of</strong>emoral Track<br />
• <strong>The</strong> Triathlon Knee patell<strong>of</strong>emoral track shares the same<br />
design as preceding Stryker total knee systems, bringing over a<br />
decade <strong>of</strong> excellent clinical performance and the industry’s<br />
lowest revision rate (0.3%) 10 to date to this knee system.<br />
Over 100,000 implanted in just two years...<br />
To learn more about the Motion, Fit and Wear <strong>of</strong> Triathlon, please visit: www.stryker.com<br />
7. Stryker test data RD-03-041 and RD-04-027.<br />
8. Stryker initiated Dynamic Computer Simulations <strong>of</strong> Passive ROM and Oxford Rig Test, Stephen Piazza, 2003.<br />
9. Churchill, D.L., Incavo, S.J., Johnson, C.C., Beynnon, B.D. <strong>The</strong> Transepicondylar Axis Approximates the Optimal<br />
Flexion Axis <strong>of</strong> the Knee, Clinical Orthopaedics. Nov. 1998 (356): 111-8.<br />
10. Robertson, O., Knutson, K., Lewold, S., Lidgren, L. <strong>The</strong> Swedish Knee Arthroplasty Register, Outcome with Special<br />
Emphasis on 1988-1997. Department <strong>of</strong> Orthopedics, University Hospital.<br />
13. Greene, K.A. Range <strong>of</strong> Motion: Early Results from the Triathlon Knee System, Stryker Literature Ref # LSA56., 2005.<br />
Triathlon<br />
Stryker Orthopaedics 325 Corporate Drive, Mahwah, NJ 07430 www.stryker.com<br />
A surgeon must always rely on his or her own pr<strong>of</strong>essional clinical judgment when deciding to use which products and/or<br />
techniques on individual patients. Stryker is not dispensing medical advice and recommends that surgeons be trained in<br />
orthopaedic surgeries before performing any surgeries.<br />
<strong>The</strong> information presented in this brochure is intended to demonstrate the breadth <strong>of</strong> Stryker product <strong>of</strong>ferings. Always refer to<br />
the package insert, product label and/or user instructions before using any Stryker product. Products may not be available in all<br />
markets. Product availability is subject to the regulatory or medical practices that govern individual markets.<br />
Please contact your Stryker representative if you have questions about the availability <strong>of</strong> Stryker products in your area.<br />
Stryker Corporation or its divisions or other corporate affiliated entities own, use or have applied for the following trademarks<br />
or service marks: Triathlon, X3, N 2 \Vac. All other trademarks are trademarks <strong>of</strong> their respective owners<br />
or holders.7<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
Experience<br />
Reserve your<br />
AAOS Annual Meeting<br />
Hotel Room on<br />
August 16th!<br />
the very best in orthopaedic education, research, and technology<br />
On Thursday, August 16, hotel room reservations<br />
for the AAOS 75th Annual Meeting become available<br />
online to AAOS Members only.<br />
Choose from a wide selection <strong>of</strong> San Francisco hotels—priced at special rates<br />
negotiated for the AAOS Annual Meeting. We know housing is important to<br />
you. Act early to take advantage <strong>of</strong> this member benefit.<br />
On October 3, Members-Only Early Registration opens online.<br />
Watch for email alerts* in advance <strong>of</strong> these opening dates, bringing you the<br />
Members Only website addresses.<br />
*Does the AAOS have your latest email address? If not, please go to www.aaos.org, click on<br />
Member Services, then My Pr<strong>of</strong>ile, then Email Address. Or just email us your address at<br />
meeting@aaos.org.<br />
AAOS 75 th Annual Meeting<br />
San Francisco, California<br />
March 5 – 9, 2008<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
<strong>The</strong> Science <strong>of</strong> Better Fit<br />
Designed with a wide range <strong>of</strong> sizing options, based on<br />
the anatomical differences <strong>of</strong> men and women. 6<br />
<strong>The</strong> Triathlon Knee System addresses smaller anatomies,<br />
<strong>of</strong>ten found in female patients, heavily concentrated in<br />
the region shown, while still accommodating larger male<br />
patients.<br />
• Broad range <strong>of</strong> size <strong>of</strong>ferings are based on an anthropometric<br />
measurement study 6 for improved interplay between implant<br />
geometry and anatomic structure for women and men.<br />
• <strong>The</strong> Triathlon design incorporates a variable aspect ratio to<br />
adequately fit the female anatomy while still accommodating the<br />
male population. 6<br />
• <strong>The</strong> many design aspects <strong>of</strong> the implant accommodate surgical<br />
realities.<br />
7-Degree Anterior Flange<br />
• <strong>The</strong> unique 7-degree anterior flange<br />
design <strong>of</strong> the Triathlon Knee System is<br />
designed to provide the flexibility to<br />
downsize the femoral component while<br />
avoiding the incidence <strong>of</strong> notching.<br />
This feature culminates in the potential<br />
to provide patients with a more<br />
customized fit.<br />
To learn more about the Motion, Fit and Wear <strong>of</strong> Triathlon, please visit: www.stryker.com<br />
6. Hitt, K., et al. Anthropometric Measurement <strong>of</strong> the Human Knee: correlation to the Sizing <strong>of</strong> Current Knee<br />
Arthroplasty Systems, JBJS, Vol. 85-A, Supplement 4, 2003.<br />
Over 100,000 implanted in just two years...<br />
Triathlon<br />
Stryker Orthopaedics 325 Corporate Drive, Mahwah, NJ 07430 www.stryker.com<br />
A surgeon must always rely on his or her own pr<strong>of</strong>essional clinical judgment when deciding to use which products and/or<br />
techniques on individual patients. Stryker is not dispensing medical advice and recommends that surgeons be trained in<br />
orthopaedic surgeries before performing any surgeries.<br />
<strong>The</strong> information presented in this brochure is intended to demonstrate the breadth <strong>of</strong> Stryker product <strong>of</strong>ferings. Always refer to<br />
the package insert, product label and/or user instructions before using any Stryker product. Products may not be available in all<br />
markets. Product availability is subject to the regulatory or medical practices that govern individual markets.<br />
Please contact your Stryker representative if you have questions about the availability <strong>of</strong> Stryker products in your area.<br />
Stryker Corporation or its divisions or other corporate affiliated entities own, use or have applied for the following trademarks<br />
or service marks: Triathlon, X3, N 2 \Vac. All other trademarks are trademarks <strong>of</strong> their respective owners<br />
or holders.7<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
Adv 32<br />
JBJS [Br] Abstracts Now Available<br />
Hip<br />
Reduction <strong>of</strong> the potential for thermal damage during hip resurfacing<br />
H. S. Gill, P. A. Campbell, D. W. Murray, and K. A. De Smet<br />
J <strong>Bone</strong> <strong>Joint</strong> Surg Br 2007 89-B: 16-20<br />
Resurfacing arthroplasty <strong>of</strong> the hip is being used increasingly<br />
as an alternative to total hip replacement, especially for young<br />
active patients. <strong>The</strong>re is concern about necrosis <strong>of</strong> the femoral<br />
head after resurfacing which can result in fracture and loosening.<br />
Most systems use a cemented femoral component, with<br />
the potential for thermal necrosis <strong>of</strong> the cancellous bone <strong>of</strong> the<br />
reamed femoral head. We used thermal probes to record temperatures<br />
close to the cement-bone interface during resurfacing<br />
arthroplasty.<br />
<strong>The</strong> maximum temperature recorded at the cement-bone<br />
interface in four cases was approximately 68°C which was<br />
higher than that reported to kill osteocytes. A modified surgical<br />
technique using insertion <strong>of</strong> a suction cannula into the lesser<br />
trochanter, generous pulsed lavage and early reduction <strong>of</strong> the<br />
joint significantly reduced the maximum recorded cancellous<br />
bone temperature to approximately 36°C in five cases (p =<br />
0.014).<br />
We recommend the modified technique since it significantly<br />
reduces temperatures at the cement-bone interface.<br />
Trauma<br />
Conservative treatment <strong>of</strong> isolated fractures <strong>of</strong> the medial malleolus<br />
D. Herscovici, Jr, J. M. Scaduto, and A. Infante<br />
J <strong>Bone</strong> <strong>Joint</strong> Surg Br 2007 89-B: 89-93<br />
Between 1992 and 2000, 57 patients with 57 isolated fractures<br />
<strong>of</strong> the medial malleolus were treated conservatively by<br />
immobilisation in a cast. <strong>The</strong> results were assessed by examination,<br />
radiography and completion <strong>of</strong> the short form-36 questionnaire<br />
and American Orthopaedic Foot and Ankle Society<br />
ankle-hindfoot score.<br />
Of the 57 fractures 55 healed without further treatment.<br />
<strong>The</strong> mean combined dorsi- and plantar flexion was 52.3° (25°<br />
to 82°) and the mean short form-36 and American Orthopaedic<br />
Foot and Ankle Society scores 48.1 (28 to 60) and 89.8 (69 to<br />
100), respectively. At review there was no evidence <strong>of</strong> medial<br />
instability, dermatological complications, malalignment <strong>of</strong> the<br />
mortise or <strong>of</strong> post-traumatic arthritis.<br />
Isolated fractures <strong>of</strong> the medial malleolus can obtain high<br />
rates <strong>of</strong> union and good functional results with conservative<br />
treatment. Operation should be reserved for bi- or trimalleolar<br />
fractures, open fractures, injuries which compromise the skin or<br />
those involving the plafond or for patients who develop painful<br />
nonunion.<br />
Knee<br />
Validation <strong>of</strong> the short-form WOMAC function scale for the evaluation<br />
<strong>of</strong> osteoarthritis <strong>of</strong> the knee<br />
K. G. Auw Yang, N. J. H. Raijmakers, A. J. Verbout, W. J. A. Dhert, and<br />
D. B. F. Saris<br />
J <strong>Bone</strong> <strong>Joint</strong> Surg Br 2007 89-B: 50-56<br />
This study validates the short-form WOMAC function<br />
scale for assessment <strong>of</strong> conservative treatment <strong>of</strong> osteoarthritis<br />
<strong>of</strong> the knee. Data were collected before treatment and six and<br />
nine months later, from 100 patients with osteoarthritis <strong>of</strong> the<br />
knee to determine the validity, internal consistency, test-retest<br />
reliability, floor and ceiling effects, and responsiveness <strong>of</strong> the<br />
short-form WOMAC function scale. <strong>The</strong> scale showed high correlation<br />
with the traditional WOMAC and other measures. <strong>The</strong><br />
internal consistency was good (Cronbach : 0.88 to 0.95) and an<br />
excellent test-retest reliability was found (Lin’s concordance correlation<br />
coefficient ( c): 0.85 to 0.94). <strong>The</strong> responsiveness was<br />
adequate and comparable to that <strong>of</strong> the traditional WOMAC<br />
(standardised response mean 0.56 to 0.44 and effect size 0.64 to<br />
0.57) and appeared not to be significantly affected by floor or<br />
ceiling effects (0% and 7%, respectively).<br />
<strong>The</strong> short-form WOMAC function scale is a valid, reliable<br />
and responsive alternative to the traditional WOMAC in the<br />
evaluation <strong>of</strong> patients with osteoarthritis <strong>of</strong> the knee managed<br />
conservatively. It is simple to use in daily practice and is therefore<br />
less <strong>of</strong> a burden for patients in clinical trials.<br />
Downloaded From: http://jbjs.org/ on 01/27/2014<br />
THE JOURNAL OF<br />
BONE AND JOINT SURGERY<br />
www.jbjs.org.uk
<strong>The</strong> Science <strong>of</strong> Reduced Wear<br />
High performance through improved design<br />
kinematics combined with X3 Advanced Bearing Technology. 1<br />
<strong>The</strong> Triathlon Knee System is designed to<br />
reduce rotational stresses, increase overall<br />
contact area, and minimize backside wear.<br />
• Through the Rotary Arc design, anatomic radius,<br />
and flared posterior condyles, the Triathlon Knee<br />
System balances conformity with constraint to<br />
mimic natural knee kinematics and the potential<br />
for enhanced wear properties. 2,3,7<br />
Wear Test Results: Triathlon Knee<br />
System with X3 versus Competitive<br />
Premium Bearing Technology 1<br />
X3 Advanced Bearing Technology<br />
• Structural Contact Fatigue Strength better than<br />
conventional polyethylene 11<br />
Locking tabs<br />
to secure wire<br />
Insert guide<br />
• Improved Wear Performance 2,3<br />
• Oxidation Resistance 4,5<br />
Same as virgin polyethylene<br />
Anti-Rotation Island<br />
designed to minimize<br />
insert micromotion<br />
and creep<br />
Improved Locking Mechanism Design<br />
• Full periphery locking rim and locking wire.<br />
• Anti-rotation island.<br />
• Reduce micromotion and promote<br />
ease <strong>of</strong> insertion. 12<br />
Over 100,000 implanted in just two years...<br />
To learn more about the Motion, Fit and Wear <strong>of</strong> Triathlon, please visit: www.stryker.com<br />
1. Stryker Orthopaedics Test Report: RD-06-013.<br />
2. Stryker Orthopaedics Triathlon CR Tibial Inserts made from X3 UHMWPE, 5530-G-409 show a 68% reduction in<br />
volumetric wear rate versus the same insert fabricated from N 2 \Vac gamma sterilized UHMWPE, 5530-P-409. <strong>The</strong> insert<br />
tested was Size 4, 9mm thick. Testing was conducted under multiaxial knee simulator (multi-station MTS knee joint<br />
simulator [a]) for five million cycles using appropriate size CoCr counterfaces, a specific type <strong>of</strong> diluted calf serum<br />
lubricant and the motion and loading conditions, representing normal walking, outlined in ISO/DIS 14243-3.<br />
Volumetric wear rates were 17.7 ± 2.2 mm 3 /10 6 cycles for standard polyethylene inserts and 5.7 ± 1.5 mm 3 /10 6 cycles for<br />
test samples. Test inserts were exposed to a gas plasma sterilization process. In vitro knee wear simulator tests have not<br />
been shown to quantitatively predict clinical wear performance.<br />
[a] A. Essner, A. Wang, C. Stark and J. H. Dumbleton. A simulator for the evaluation <strong>of</strong> total knee replacement<br />
wear, 5th World Biomaterials Congress, Toronto, Canada, May 1996, pg 580.<br />
3. Stryker Orthopaedics Triathlon PS Tibial Inserts made <strong>of</strong> X3 UHMWPE, 5532-G-409 show a 64% reduction in<br />
volumetric wear rate versus the same insert fabricated from N 2 \Vac gamma sterilized UHMWPE, 5532-P-409. <strong>The</strong> insert<br />
tested was Size 4, 9mm thick. Testing was conducted under multiaxial knee simulator (multi-station MTS knee joint<br />
simulator) for five million cycles using a size 7 CoCr counterfaces, a specific type <strong>of</strong> diluted calf serum lubricant and<br />
literature or fluoroscopy based motion and loading conditions representing stair climbing [b,c]. Volumetric wear rates<br />
were 3.6 ± 0.61 mm 3 /10 6 cycles for standard polyethylene inserts and were 1.3 ± 0.44 mm 3 /10 6 cycles for test samples.<br />
Test inserts were exposed to a gas plasma sterilization process. In vitro knee wear simulator tests have not been shown to<br />
quantitatively predict clinical wear performance.<br />
[b] R. Riener, M. Rabuffetti and C. Frigo. Stair ascent and descent at different inclinations, Gait and Posture 15:<br />
2002, pp. 32-44.<br />
[c] JB. Morrison. Function <strong>of</strong> the knee joint in various activities, Bio-medical Engineering, 4: 1969, pp. 573-580.<br />
4. X3 UHMWPE maintains mechanical properties after accelerated oxidative aging. No statistical difference was found for<br />
Tensile Yield Strength, Ultimate Tensile Strength and Elongation as measured per ASTM D638 before and after exposure<br />
to ASTM F2003 accelerated aging (5 Atmospheres (ATM) <strong>of</strong> oxygen at 70°C for 14 days). Tensile Yield Strength was 23.5<br />
± 0.3 MPa and 23.6 ± 0.2 MPa, Ultimate Tensile Strength was 56.7 ± 2.1 MPa and 56.3 ± 2.3 MPa, and Elongation was<br />
267 ± 7% and 266 ± 9% before and after accelerated oxidative aging, respectively.<br />
5. X3 UHMWPE resists the effects <strong>of</strong> oxidation. No statistical difference was found for Tensile Yield Strength, Ultimate<br />
Tensile Strength, Elongation, Crystallinity and Density as measured per ASTM D638, D3417 and D1505 before and after<br />
ASTM F2003 accelerated aging (5 ATM <strong>of</strong> oxygen at 70°C for 14 days). Tensile Yield Strength was 23.5 ± 0.3 MPa and<br />
23.6 ± 0.2 MPa, Ultimate Tensile Strength was 56.7 ± 2.1 MPa and 56.3 ± 2.3 MPa, Elongation was 267 ±7%and266±<br />
9%, Crystallinity was 61.7 ± 0.6% and 61.0 ± 0.5%, and Density was 939.2 ± 0.1 kg/m3 before and after accelerated<br />
oxidative aging, respectively.<br />
7. Stryker test data RD-03-041 and RD-04-027.<br />
11. Contact fatigue testing was conducted on accelerated aged sequentially crosslinked and annealed (X3) UHMWPE and<br />
control materials. Testing involved translating a 22mm CoCr femoral head across 5mm thick samples at 0.5 Hz under a<br />
200 N load for a contact stress <strong>of</strong> 62 MPa. Control materials were nitrogen packed and gamma-radiation sterilized and<br />
air packed and gamma-radiation sterilized UHMWPE’s. X3 samples were unsterilized. All contact fatigue samples were<br />
ASTM F2003 accelerated aged (5 ATM <strong>of</strong> oxygen at 70°C for 14 days). Testing induced cracking in control materials in as<br />
little as 0.5 million cycles while proposed material samples showed no signs <strong>of</strong> pitting, cracking (surface or subsurface)<br />
after 5 million cycles. Control material cracks progressed to gross material loss (delamination).<br />
<strong>The</strong> results <strong>of</strong> the in-vitro delamination tests have not been shown to correlate with clinical delamination mechanisms.<br />
12. Stryker Test Report. Multi-Axis Dynamic Fatigue Test MTP101.0-02.<br />
Triathlon<br />
Stryker Orthopaedics 325 Corporate Drive, Mahwah, NJ 07430 www.stryker.com<br />
A surgeon must always rely on his or her own pr<strong>of</strong>essional clinical judgment when deciding to use which products and/or<br />
techniques on individual patients. Stryker is not dispensing medical advice and recommends that surgeons be trained in<br />
orthopaedic surgeries before performing any surgeries.<br />
<strong>The</strong> information presented in this brochure is intended to demonstrate the breadth <strong>of</strong> Stryker product <strong>of</strong>ferings. Always refer to<br />
the package insert, product label and/or user instructions before using any Stryker product. Products may not be available in all<br />
markets. Product availability is subject to the regulatory or medical practices that govern individual markets.<br />
Please contact your Stryker representative if you have questions about the availability <strong>of</strong> Stryker products in your area.<br />
Stryker Corporation or its divisions or other corporate affiliated entities own, use or have applied for the following trademarks<br />
or service marks: Triathlon, X3, N 2 \Vac. All other trademarks are trademarks <strong>of</strong> their respective owners<br />
or holders.7<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
2006 Corporate Associates<br />
Platinum Level ($200,000 & above)<br />
Gold Level<br />
($100,000 - $199,999)<br />
Silver Level<br />
($50,000 - $99,999)<br />
Bronze<br />
Level<br />
($10,000<br />
- $49,999)<br />
Copper<br />
Level<br />
($1,000 -<br />
$9,999)<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
LINK ® BetaCone TM<br />
Hip Prosthesis System<br />
This device is deared for use without bone cement in the United States.<br />
LINK ORTHOPAEDICS · 300 Roundhill Dr. · Rockaway, NJ 07866 · USA<br />
Phone 1-973-625-1333 (U.S. and Canada) · Phone 1-973-625-4445<br />
e-mail: link@linkorthopaedics.com<br />
Distributed in U.S. and Canada by Exactech, Inc. · FL 32653 Gainesville · Phone 1-800-392-2832<br />
Manufactured: WALDEMAR LINK GmbH & Co. KG · www.linkhh.de · Germany<br />
www.betacone.com<br />
Next<br />
generation<br />
hip prosthesis<br />
providing excellent<br />
biomechanics<br />
to reduce “stress-shielding“<br />
Time has come for change!<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
“ DYONICS ELECTROBLADE Resector makes<br />
quick work <strong>of</strong> subacromial decompressions.”<br />
LAWRENCE LEMAK, MD<br />
LEMAK Sports Medicine Institute, Birmingham, Alabama<br />
<br />
Exclusively from Smith & Nephew, the DYONICS ELECTROBLADE Resector<br />
streamlines subacromial decompressions by delivering simultaneous s<strong>of</strong>t<br />
tissue resection and hemostasis. This reduces instrument exchanges,<br />
minimizes bleeding and enhances visualization. Please contact us to learn<br />
more about how DYONICS products can simplify your procedures.<br />
Endoscopy<br />
Smith & Nephew, Inc.<br />
150 Minuteman Road<br />
Andover, MA 01810<br />
www.smith-nephew.com<br />
T +1 978 749 1000 US<br />
Trademark <strong>of</strong> Smith & Nephew.<br />
Certain marks are Reg US Pat and TM Office.<br />
©2007 Smith & Nephew, Inc. All rights reserved<br />
Printed in USA 06/07 2071 Rev. A<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
Dislocation<br />
concerns?<br />
Lifestyle Recovery <br />
Technologies<br />
Helping you to help patients maximize ROM and<br />
enhance stability.<br />
LFIT Anatomic<br />
CoCr Femoral<br />
Head with<br />
X3 Liner<br />
UHRBipolar<br />
Femoral Head<br />
Secur-Fit Max<br />
Femoral Hip<br />
Stem<br />
Trident<br />
Constrained<br />
Insert<br />
Formore information,contactyourlocal Stryker sales representative at<br />
1-866-447-3627 or visit www.stryker.com<br />
Lifestyle Recovery Technologies, LFIT AnatomicCoCr Femoral Head,<br />
X3 Liner,UHRBipolar Head,Trident Constrained Insert<br />
Stryker Orthopaedics<br />
325 Corporate Drive, Mahwah, NJ 07430<br />
Asurgeon is the best person to decide with the patient which treatments andproducts are right for them.<br />
Surgeons must always rely on their ownclinicaljudgement when deciding which treatments andprocedureto<br />
use with patients. Copyright © 2006 Stryker. Stryker Corporation orits divisions or other corporate affiliated<br />
entities own, use or have appliedfor the following trademarks:LFIT, Lifestyle Recovery, Secur-Fit, Stryker,<br />
Trident, UHR, X3.<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
IMPORTANT SAFETY INFORMATION:<br />
*Because <strong>of</strong> its clotting mechanism, Thrombin-JMI ® must not be injected or otherwise allowed to enter large blood vessels. Extensive intravascular clotting or even<br />
death may result. Thrombin-JMI ® should be given to a pregnant woman only if clearly indicated. Safety and efficacy in children have not been established.<br />
<strong>The</strong> use <strong>of</strong> topical bovine thrombin preparations has occasionally been associated with abnormalities in hemostasis ranging from asymptomatic alterations in<br />
laboratory determinations, such as prothrombin time (PT) and partial thromboplastin time (PTT), to severe bleeding or thrombosis which rarely have been fatal.<br />
<strong>The</strong>se hemostatic effects appear to be related to the formation <strong>of</strong> antibodies against bovine thrombin and/or factor V which in some cases may cross react with<br />
human factor V, potentially resulting in factor V deficiency. Repeated clinical applications <strong>of</strong> topical bovine thrombin increase the likelihood that antibodies against<br />
thrombin and/or factor V may be formed. Consultation with an expert in coagulation disorders is recommended if a patient exhibits abnormal coagulation<br />
laboratory values, abnormal bleeding or abnormal thrombosis following the use <strong>of</strong> topical thrombin. Any interventions should consider the immunologic basis <strong>of</strong><br />
this condition. Patients with antibodies to bovine thrombin preparations should not be re-exposed to these products.<br />
Please see brief summary <strong>of</strong> full Prescribing Information for Thrombin-JMI ® on adjacent page.<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
Gets to the Site<br />
<strong>of</strong> the Bleeding<br />
Your Trusted Thrombin-on-the-Spot for Fast, Active Hemostasis<br />
•Hemostasis achieved within 3 minutes in 71% <strong>of</strong> patients undergoing spinal surgery who were treated with<br />
Thrombin-JMI ® -soaked Absorbable Gelatin Sponge, USP 1<br />
•A biologically active agent that works directly at the end <strong>of</strong> the coagulation cascade 2<br />
Multiple Delivery Options for Hemostasis—<br />
Where You Want It, When You Need It<br />
• Thrombin-JMI ® Pump Spray Kit to cover broad oozing areas<br />
• Thrombin-JMI ® Syringe Spray Kit for difficult-to-reach bleeding sites*<br />
•Thrombin-JMI ® Vials for direct application or use in conjunction with certain passive hemostatic agents<br />
Thrombin-JMI ® is indicated as an aid to hemostasis whenever oozing blood and minor bleeding from capillaries<br />
and small venules is accessible. In various types <strong>of</strong> surgery, solutions <strong>of</strong> Thrombin-JMI ® may be used in conjunction<br />
with an Absorbable Gelatin Sponge, USP for hemostasis.<br />
Makes Contact. Takes Action.<br />
Thrombin-JMI is a registered trademark <strong>of</strong> King Pharmaceuticals Research and Development, Inc., a wholly owned subsidiary <strong>of</strong> King Pharmaceuticals ® , Inc.<br />
Copyright © 2006 King Pharmaceuticals ® , Inc. All rights reserved. JMI4276 12/2006<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
THROMBIN, TOPICAL U.S.P.<br />
(BOVINE ORIGIN)<br />
THROMBIN-JMI ®<br />
BRIEF SUMMARY <strong>of</strong> Full Prescribing Information<br />
<br />
Thrombin, Topical (Bovine) must not be injected! Apply on<br />
the surface <strong>of</strong> bleeding tissue.<br />
INDICATIONS AND USAGE<br />
THROMBIN-JMI ® is indicated as an aid to hemostasis whenever<br />
oozing blood and minor bleeding from capillaries and small<br />
venules is accessible.<br />
In various types <strong>of</strong> surgery, solutions <strong>of</strong> THROMBIN-JMI ® may<br />
be used in conjunction with an Absorbable Gelatin Sponge, USP<br />
for hemostasis.<br />
CONTRAINDICATIONS<br />
THROMBIN-JMI ® is contraindicated in persons known to be sensitive<br />
to any <strong>of</strong> its components and/or to material <strong>of</strong> bovine origin.<br />
WARNING<br />
<strong>The</strong> use <strong>of</strong> topical bovine thrombin preparations has<br />
occasionally been associated with abnormalities in<br />
hemostasis ranging from asymptomatic alterations in<br />
laboratory determinations, such as prothrombin time<br />
(PT) and partial thromboplastin time (PTT), to severe<br />
bleeding or thrombosis which rarely have been fatal.<br />
<strong>The</strong>se hemostatic effects appear to be related to the formation<br />
<strong>of</strong> antibodies against bovine thrombin and/or factor<br />
V which in some cases may cross react with human<br />
factor V, potentially resulting in factor V deficency.<br />
Repeated clinical applications <strong>of</strong> topical bovine thrombin<br />
increase the likelihood that antibodies against<br />
thrombin and/or factor V may be formed. Consultation<br />
with an expert in coagulation disorders is recommended<br />
if a patient exhibits abnormal coagulation laboratory values,<br />
abnormal bleeding, or abnormal thrombosis following<br />
the use <strong>of</strong> topical thrombin. Any interventions should<br />
consider the immunologic basis <strong>of</strong> this condition.<br />
Patients with antibodies to bovine thrombin preparations<br />
should not be re-exposed to these products.<br />
Because <strong>of</strong> its action in the clotting mechanism, THROM-<br />
BIN-JMI ® must not be injected or otherwise allowed to enter<br />
large blood vessels. Extensive intravascular clotting and<br />
even death may result.<br />
PRECAUTIONS<br />
General — Consult the Absorbable Gelatin Sponge, USP labeling<br />
for complete information for use prior to utilizing the thrombin<br />
saturated sponge procedure.<br />
Pregnancy — Category C – Animal reproduction studies have<br />
not been conducted with THROMBIN-JMI ® . It is also not known<br />
whether THROMBIN-JMI ® can cause fetal harm when administered<br />
to a pregnant woman or can affect reproduction capacity.<br />
THROMBIN-JMI ® should be given to a pregnant woman only if<br />
clearly indicated.<br />
Pediatric Use — Safety and effectiveness in children have not<br />
been established.<br />
ADVERSE REACTIONS<br />
Allergic reactions may be encountered in persons known to be<br />
sensitive to bovine materials. Inhibitory antibodies which interfere<br />
with hemostasis may develop in a small percentage <strong>of</strong><br />
patients. See Warning.<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
Distributed By:<br />
Jones Pharma Incorporated, Bristol, VA 24201<br />
Manufactured by:<br />
GenTrac, Incorporated, Middleton, Wisconsin 53562<br />
U.S. License No. 977<br />
Rev. 5/05<br />
Jones Pharma Incorporated and GenTrac,<br />
Incorporated Jones Pharma are Incorporated wholly owned subsidiaries and GenTrac, <strong>of</strong><br />
King Incorporated Pharmaceuticals are wholly ® , Inc. owned subsidiaries <strong>of</strong><br />
King Pharmaceuticals ® , Inc.<br />
References: 1. Renkens KL Jr, Payner TD, Leipzig TJ, et al. A<br />
multicenter, prospective, randomized trial evaluating a new<br />
hemostatic agent for spinal surgery. Spine. 2001;26:1645-<br />
1650. 2.Thrombin-JMI [package insert]. Middleton, Wis:<br />
GenTrac, Inc; 2005.<br />
<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
Downloaded From: http://jbjs.org/ on 01/27/2014
<strong>The</strong> Main Event<br />
<strong>The</strong> comprehensive meeting on hip resurfacing<br />
October 25 - 27, 2007<br />
Loews Miami Beach Hotel<br />
Miami, Florida<br />
Register online at www.orthomeetings.com<br />
Chairmen<br />
Derek McMinn, FRCS<br />
Edgbaston, Birmingham<br />
United Kingdom<br />
Ronan Treacy, FRCS<br />
Edgbaston, Birmingham<br />
United Kingdom<br />
Trademark <strong>of</strong> Smith & Nephew. Reg. US Pat. & TM Off.<br />
10 Years<br />
1997-2007<br />
BIRMINGHAM HIP Resurfacing<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
Downloaded From: http://jbjs.org/ on 01/27/2014
Downloaded From: http://jbjs.org/ on 01/27/2014
® <br />
<br />
® <br />
<br />
<br />
<br />
® <br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
® <br />
<br />
<br />
® <br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
See brief summary on page 18<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
® ® <br />
® <br />
® <br />
® <br />
<br />
<br />
® <br />
® <br />
® <br />
<br />
<br />
® <br />
<br />
<br />
® <br />
<br />
<br />
® <br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
® <br />
<br />
<br />
<br />
Photo © www.KarlGrobl.com<br />
<br />
<br />
<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
Take the guesswork out <strong>of</strong><br />
shoulder reconstruction.<br />
Only the HemiCAP ® system can recreate both the non-spherical and spherical curvatures<br />
<strong>of</strong> the humeral head using 3D intraoperative mapping without changing<br />
head height, version or tension <strong>of</strong> the s<strong>of</strong>t tissue envelope.<br />
Map it and HemiCAP ®<br />
it!<br />
X-rays courtesy <strong>of</strong> John Macy, M.D., Burlington, VT, USA<br />
28 forge parkway, franklin, ma 02038 tel +1 508 520 3003 fax +1 508 528 4604 web arthrosurface.com<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
Adv 48<br />
JBJS [Br] Abstracts Now Available<br />
Trauma<br />
<strong>The</strong> undiagnosed Essex-Lopresti injury<br />
P. Jungbluth, T. M. Frangen, S. Arens, G. Muhr; and T. Kälicke<br />
From BG-Kliniken Bergmannsheil Bochum, Bochum, Germany<br />
J <strong>Bone</strong> <strong>Joint</strong> Surg (Br) 2006;88-B:1629-33<br />
<strong>The</strong> Essex-Lopresti injury is rare. It consists <strong>of</strong> fracture <strong>of</strong> the<br />
head <strong>of</strong> the radius, rupture <strong>of</strong> the interosseous membrane and<br />
disruption <strong>of</strong> the distal radioulnar joint. <strong>The</strong> injury is <strong>of</strong>ten<br />
missed because attention is directed towards the fracture <strong>of</strong> the<br />
head <strong>of</strong> the radius. We present a series <strong>of</strong> 12 patients with a<br />
mean age <strong>of</strong> 44.9 years (26 to 54), 11 <strong>of</strong> whom were treated<br />
surgically at a mean <strong>of</strong> 4.6 months (1 to 16) after injury and<br />
the other after 18 years. <strong>The</strong>y were followed up for a mean <strong>of</strong><br />
29.2 months (2 to 69). Ten patients had additional injuries<br />
to the forearm or wrist, which made diagnosis more difficult.<br />
Replacement <strong>of</strong> the head <strong>of</strong> the radius was carried out in ten<br />
patients and the Sauve-Kapandji procedure in three. Patients<br />
were assessed using standard outcome scores. <strong>The</strong> mean postoperative<br />
Disabilities <strong>of</strong> the Arm, Shoulder and Hand score was<br />
55 (37 to 83), the mean Morrey Elbow Performance score was<br />
72.2 (39 to 92) and the mean Mayo wrist score was 61.3 (35 to<br />
80). <strong>The</strong> mean grip strength was 68.5% (39.6% to 91.3%) <strong>of</strong><br />
the unaffected wrist.<br />
Most <strong>of</strong> the patients (10 <strong>of</strong> 12) were satisfied with their<br />
operation and in 11 the pain was relieved. When treating the<br />
chronic Essex-Lopresti injury, we recommend accurate realignment<br />
<strong>of</strong> the radius and ulna and replacement <strong>of</strong> the head <strong>of</strong> the<br />
radius. If this fails a Sauve-Kapandji procedure to arthrodese<br />
the distal radioulnar joint should be undertaken to stabilise the<br />
forearm while maintaining mobility.<br />
Hip<br />
going resurfacing (57%; 36) had an <strong>of</strong>fset ratio 0.15 pre-operatively<br />
and required greater correction <strong>of</strong> <strong>of</strong>fset at operation than<br />
the rest <strong>of</strong> the group. In the non-arthritic hips the mean <strong>of</strong>fset<br />
ratio was 0.137 (0.04 to 0.23), with the <strong>of</strong>fset ratio correlating<br />
negatively to an increasing angle. An <strong>of</strong>fset ratio 0.15 had a<br />
9.5-fold increased relative risk <strong>of</strong> having an angle 50.5°. Most<br />
hips undergoing resurfacing have an abnormal femoral head/<br />
neck <strong>of</strong>fset, which is best assessed in the sagittal plane.<br />
Knee<br />
Determining the rotational alignment <strong>of</strong> the tibial component at<br />
total knee replacement<br />
A COMPARISON OF TWO TECHNIQUES<br />
M. Ikeuchi, N. Yamanaka, Y. Okanoue, E. Ueta, and T. Tani<br />
J <strong>Bone</strong> <strong>Joint</strong> Surg Br 2007 89-B: 45-49<br />
We prospectively assessed the benefits <strong>of</strong> using either a range<strong>of</strong>-movement<br />
technique or an anatomical landmark method to<br />
determine the rotational alignment <strong>of</strong> the tibial component during<br />
total knee replacement. We analysed the cut proximal tibia<br />
intraoperatively, determining anteroposterior axes by the range<strong>of</strong>-movement<br />
technique and comparing them with the anatomical<br />
anteroposterior axis.<br />
We found that the range-<strong>of</strong>-movement technique tended to<br />
leave the tibial component more internally rotated than when<br />
anatomical landmarks were used. In addition, it gave widely<br />
variable results (mean 7.5°; 2° to 17°), determined to some<br />
extent by which posterior reference point was used. Because <strong>of</strong><br />
the wide variability and the possibilities for error, we consider<br />
that it is inappropriate to use the range-<strong>of</strong>-movement technique<br />
as the sole method <strong>of</strong> determining alignment <strong>of</strong> the tibial component<br />
during total knee replacement.<br />
<strong>The</strong> femoral head/neck <strong>of</strong>fset and hip resurfacing<br />
P. E. Beaulé, N. Harvey, E. Zaragoza, M. J. Le Duff, and F. J. Dorey<br />
J <strong>Bone</strong> <strong>Joint</strong> Surg Br 2007 89-B: 9-15<br />
Because the femoral head/neck junction is preserved in hip<br />
resurfacing, patients may be at greater risk <strong>of</strong> impingement,<br />
leading to abnormal wear patterns and pain. We assessed femoral<br />
head/neck <strong>of</strong>fset in 63 hips undergoing metal-on-metal hip<br />
resurfacing and in 56 hips presenting with non-arthritic pain<br />
secondary to femoroacetabular impingement. Most hips under-<br />
Downloaded From: http://jbjs.org/ on 01/27/2014<br />
THE JOURNAL OF<br />
BONE AND JOINT SURGERY<br />
www.jbjs.org.uk
Ankle Fusion has an<br />
Improved Alternative<br />
<strong>The</strong> 4th generation Agility LP Ankle is a<br />
new member <strong>of</strong> the Agility Total Ankle System<br />
that provides:<br />
• New cutting blocks to provide precise,<br />
reproducible tibial, fibular and talar cuts<br />
• Broader cortical coverage <strong>of</strong> the cut talus<br />
• System has 90% survivorship with revision<br />
option 1<br />
• Over 20 years <strong>of</strong> clinical experience 2,3,4<br />
For more information, contact<br />
your local DePuy representative.<br />
© 2007 DePuy Orthopaedics, Inc. All rights reserved.<br />
References:<br />
1. Data on file at DePuy Orthopaedics, Inc.<br />
2. Kronemyer, B.: Results <strong>of</strong> First 100 Agility Total Ankle Arthroplasties<br />
Encouraging. Orthopedics Today, July 1997, Vol. 17, No. 7: 16-17.<br />
3. Alvine, F. G.: Total Ankle Arthroplasty: New Concepts and Approach.<br />
Contemporary Orthopaedics, April 1991, Vol. 22, No. 4: 397-403.<br />
4. Pyevich, M. T.; Saltzman, C. L. and Callaghan, J. J.: Total Ankle Arthroplasty:<br />
A Unique Design. JBJS, October 1998, Vol. 80-A, No. 10: 1410-1420.<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
Features <strong>of</strong> BONE FOAM<br />
• Radiolucent for<br />
C-Arm imaging<br />
• Saves 10-20 minutes each case<br />
• Consistent and<br />
stable<br />
• Strategic design for each extremity<br />
• 100% latex freee<br />
• Durable stain resistant coating<br />
• Sterile bags available ailable to fit most pieces<br />
• Fire retardant<br />
• Washable for infection control<br />
TO<br />
ORDER<br />
612.338.1400<br />
www.bonefoam. com<br />
sales@excelmedsolutions.com<br />
lmedsolutions.co<br />
om<br />
Before<br />
BONE FOAM<br />
BONE FOAM<br />
Before<br />
Upper Extremity<br />
Block & Wedge<br />
ORIF <strong>of</strong> Distal Humerus,<br />
Olecranon, Ulna<br />
"<strong>The</strong> upper extremity<br />
pieces have an <strong>of</strong>fset<br />
down arm tunnel that<br />
makes it perfect for C-Arm<br />
imaging."<br />
Upper Extremity Block<br />
ORIF <strong>of</strong> Distal Humerus<br />
and Olecranon<br />
"<strong>The</strong> upper extremity ty<br />
block helps to reduce and<br />
hold reduction through<br />
surgery." ry."<br />
Before<br />
BONE FOAM<br />
BONE FOAM<br />
Leg Cradle<br />
Cylindrical i<br />
Bumps<br />
ORIF <strong>of</strong> Tibial Plateau<br />
and Distal Femur<br />
"<strong>Bone</strong> Foam cylinders<br />
help me quickly bump<br />
and prop up any<br />
extremity."<br />
<strong>The</strong> Ramp/Leg Elevator<br />
ORIF <strong>of</strong> Patella, Tibia, Ankle,<br />
Midfoot ot and Forefoot oot FX<br />
"<strong>The</strong> leg Elevator helps me<br />
save time by positioning oning<br />
and stabilizing the<br />
operative leg above<br />
the<br />
nonoperative leg so<br />
lateral<br />
C-Arm Imaging is<br />
consistant and fast."<br />
Before<br />
BONE FOAM<br />
Before<br />
Retrograde<br />
Femoral Nailing<br />
Small, Medium, Large:<br />
To get the right angle<br />
Knee Wedges<br />
for positioning n<br />
and<br />
Tibial Nailing<br />
reduction.<br />
"With the acute knee<br />
wedge, the forgiveness <strong>of</strong><br />
the foam cradles the knee<br />
www.bonefoam.com<br />
to give the leg more<br />
stability when inserting a<br />
US Patent<br />
tibial nail."<br />
Leg Tunnel<br />
Posteriolateral <strong>Bone</strong><br />
Grafting and ORIF <strong>of</strong><br />
Calcaneal FX, Pelvic FX,<br />
Lateral Positioning<br />
"<strong>The</strong> leg tunnel creates<br />
a<br />
stable, flat, predictable<br />
surface on which to work,<br />
with plenty <strong>of</strong> mobility for<br />
imaging away from the<br />
down leg."<br />
a<br />
<strong>Bone</strong> Foam TM<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
Why One Million<br />
Gamma Nails<br />
Have Been Implanted<br />
Worldwide<br />
First †<br />
Pioneered by visionaries<br />
Finest<br />
Improved through experience<br />
Preferred ††<br />
Most trusted and implanted device <strong>of</strong> its type<br />
Surgeons have embraced Stryker’s Gamma<br />
technology and have implanted over one<br />
million worldwide.* This extraordinary<br />
statistic speaks to the trust surgeons across<br />
the globe place in Gamma Nails, and to<br />
the Gamma Nail innovators who strive<br />
for excellence.<br />
Nearly two decades ago, Stryker introduced<br />
the first † generation <strong>of</strong> Gamma Nail<br />
to the world market. Today, the third<br />
generation intramedullary sliding lag<br />
screw nail continues to define the industry<br />
standard.<br />
Gamma Locking Nail System<br />
<strong>The</strong> unmistakable advantages <strong>of</strong> the<br />
Gamma Nail Family<br />
• Over 17 years <strong>of</strong> clinical experience<br />
• New smaller implant with streamlined<br />
instrumentation<br />
• Minimally invasive surgery with the<br />
possibility for less bone removal<br />
• Three entry options for beginning<br />
the procedure<br />
• Potential for earlier weight bearing**<br />
Stryker is proud to work side-by-side with<br />
trauma and orthopaedic surgeons to herald<br />
in the future <strong>of</strong> orthopaedic fracture<br />
treatment.<br />
To see a Live Gamma3 procedure performed<br />
on a cadaver by Drs. Christopher Born,<br />
James Maxey and Robert Probe, please<br />
visit www.or-live.com/Gamma3/1458<br />
For more information, visit<br />
www.stryker.com/gamma or contact<br />
your Stryker Trauma Representative.<br />
* Includes Gamma, Gamma-Ti, and Gamma3<br />
** Early weight bearing as appropriate for the patient<br />
and determined by the Surgeon. References available<br />
upon request.<br />
† Based on trademark filing with the United States Patent<br />
and Trademark Office, US Registration # 2819616.<br />
†† Millennium Research Group, US Markets for Trauma<br />
Devices, February 2005, Intramedullary Hip Screw<br />
Market.<br />
Stryker Orthopaedics 325 Corporate Drive, Mahwah, NJ 07430 www.stryker.com<br />
A surgeon is the best person to decide with the patient which treatments and products are right for them. Surgeons must always rely on their<br />
own clinical judgment when deciding which treatments and procedures to use with patients. Copyright © 2007 Stryker. All rights reserved.<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
Downloaded From: http://jbjs.org/ on 01/27/2014
Downloaded From: http://jbjs.org/ on 01/27/2014
T-Pin for Distal Radius Fracture Fixation<br />
FLOSEAL [Hemostatic Matrix] Indications<br />
FLOSEAL [Hemostatic Matrix] is indicated in surgical<br />
procedures (other than ophthalmic) as an adjunct to<br />
hemostasis when control <strong>of</strong> bleeding by ligature or<br />
conventional procedures is ineffective or impractical.<br />
Important Safety Information<br />
FLOSEAL must not be injected into blood vessels, or<br />
allowed to enter blood vessels. Do not apply in the<br />
absence <strong>of</strong> active bleeding. Extensive intravascular<br />
clotting and even death may result.<br />
Do not use FLOSEAL in the closure <strong>of</strong> skin incisions<br />
because it may interfere with the healing <strong>of</strong> the<br />
skin edges.<br />
Do not use FLOSEAL in patients with known allergies to<br />
materials <strong>of</strong> bovine origin.<br />
FLOSEAL is made from human plasma. It may carry a<br />
risk <strong>of</strong> transmitting infectious agents, e.g., viruses, and<br />
theoretically, the Creutzfeldt-Jakob disease (CJD) agent.<br />
RX only: For safe and proper use <strong>of</strong> this device, please<br />
refer to full device Instructions For Use.<br />
<br />
Mini-incisions<br />
Local anesthesia<br />
Placed over guide wires<br />
Allows early wrist ROM<br />
Break-<strong>of</strong>f driver/easy insertion<br />
Enrolling surgeon consultants<br />
Technique guide available at<br />
www.unionsurgical.com<br />
834 Chestnut Street<br />
Suite G-114<br />
Philadelphia, PA 19107<br />
(215) 925-5208 Phone<br />
tpin@unionsurgical.com<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
IN THE TREATMENT OF OSTEOARTHRITIS (OA) KNEE PAIN<br />
NO VISCOSUPPLEMENT<br />
STANDS UP TO PAIN BETTER<br />
SYNVISC® (hylan G-F 20) is indicated for the treatment <strong>of</strong> pain in osteoarthritis (OA)<br />
<strong>of</strong> the knee in patients who have failed to respond adequately to conservative<br />
nonpharmacologic therapy and simple analgesics,eg,acetaminophen.<br />
In clinical trials,the most commonly reported adverse events were transient local<br />
pain,swelling,and/or effusion in the injected knee.In some cases,these symptoms<br />
have been extensive.Other side effects such as rash have been reported rarely.<br />
SYNVISC is contraindicated in patients with known hypersensitivity to hyaluronan<br />
Please see accompanying Prescribing Information.<br />
products or patients with infections in or around the knee.Use caution when using<br />
SYNVISC in patients allergic to avian proteins,feathers,or egg products;who have<br />
evidence <strong>of</strong> venous or lymphatic stasis in the leg to be treated; or who have severe<br />
inflammation in the knee joint to be treated.Patients should be advised to avoid<br />
strenuous or prolonged weight-bearing activities after treatment.Strict<br />
adherence to aseptic technique must be followed to avoid joint infection.<strong>The</strong> safety<br />
and effectiveness <strong>of</strong> SYNVISC in children and in pregnant or lactating women have<br />
not been established.It is unknown whether SYNVISC is excreted in human milk.<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
UNIQUE NATIONAL<br />
HCPCS CODE<br />
Q4084<br />
BRIEF SUMMARYFOR THE PHYSICIAN (CONSULT PACKAGE INSERT FOR FULL PRODUCT INFORMATION)<br />
CAUTION: Federal law restricts this device to sale by or on the order <strong>of</strong> a physician (or properly<br />
licensed practitioner).<br />
INDICATIONS Synvisc is indicated for the treatment <strong>of</strong> pain in osteoarthritis (OA) <strong>of</strong> the knee in patients<br />
who have failed to respond adequately to conservative nonpharmacologic therapy and simple analgesics,<br />
e.g., acetaminophen.<br />
CONTRAINDICATIONS • Do not administer to patients with known hypersensitivity (allergy) to hyaluronan<br />
(sodium hyaluronate) preparations. • Do not inject Synvisc in the knees <strong>of</strong> patients having knee joint<br />
infections or skin diseases or infections in the area <strong>of</strong> the injection site.<br />
WARNINGS • Do not concomitantly use disinfectants containing quaternary ammonium salts for skin<br />
preparation because hyaluronan can precipitate in their presence. • Do not inject Synvisc extra-articularly<br />
or into the synovial tissues and capsule. Local and systemic adverse events, generally in the area <strong>of</strong> the<br />
injection, have occurred following extra-articular injection <strong>of</strong> Synvisc. • Intravascular injections <strong>of</strong> Synvisc<br />
may cause systemic adverse events.<br />
PRECAUTIONS General • <strong>The</strong> effectiveness <strong>of</strong> a single treatment cycle <strong>of</strong> less than three injections <strong>of</strong><br />
Synvisc has not been established. • <strong>The</strong> safety and effectiveness <strong>of</strong> Synvisc in locations other than the<br />
knee and for conditions other than osteoarthritis have not been established. • Do not inject anesthetics or<br />
other medications into the knee joint during Synvisc therapy. Such medications may dilute Synvisc and<br />
affect its safety and effectiveness. • Use caution when injecting Synvisc into patients who are allergic to<br />
avian proteins, feathers, and egg products. • <strong>The</strong> safety and effectiveness <strong>of</strong> Synvisc in severely inflamed<br />
knee joints have not been established. • Strict aseptic administration technique must be followed.<br />
• STERILE CONTENTS. <strong>The</strong> syringe is intended for single use. <strong>The</strong> contents <strong>of</strong> the syringe must be used<br />
immediately after its packaging is opened. Discard any unused Synvisc. • Do not use Synvisc if package<br />
is opened or damaged. Store in original packaging (protected from light) at room temperature below<br />
86°F (30°C). DO NOT FREEZE. • Remove synovial fluid or effusion before each Synvisc injection.<br />
• Synvisc should be used with caution when there is evidence <strong>of</strong> lymphatic or venous stasis in that leg.<br />
Information for Patients • Provide patients with a copy <strong>of</strong> the Patient Labeling prior to use. • Transient<br />
pain, swelling and/or effusion <strong>of</strong> the injected joint may occur after intra-articular injection <strong>of</strong> Synvisc.<br />
In some cases the effusion may be considerable and can cause pronounced pain; cases where swelling<br />
is extensive should be discussed with the physician. • As with any invasive joint procedure, it is<br />
recommended that the patient avoid any strenuous activities or prolonged weight-bearing activities<br />
such as jogging or tennis following the intra-articular injection.<br />
Use in Specific Populations •Pregnancy: <strong>The</strong> safety and effectiveness <strong>of</strong> Synvisc have not been<br />
established in pregnant women. •Nursing mothers: It is not known if Synvisc is excreted in human milk.<br />
<strong>The</strong> safety and effectiveness <strong>of</strong> Synvisc have not been established in lactating women. • <strong>The</strong> safety and<br />
effectiveness <strong>of</strong> Synvisc have not been established in children.<br />
ADVERSE EVENTS<br />
Adverse Events Involving the Injected <strong>Joint</strong><br />
Clinical Trials: A total <strong>of</strong> 511 patients (559 knees) received 1771 injections in seven clinical trials <strong>of</strong><br />
Synvisc. <strong>The</strong>re were 39 reports in 37 patients (2.2% <strong>of</strong> injections, 7.2% <strong>of</strong> patients) <strong>of</strong> knee pain and/or<br />
swelling after these injections. Ten patients (10 knees) were treated with arthrocentesis and removal <strong>of</strong><br />
joint effusion. Two additional patients (two knees) received treatment with intra-articular steroids. Two<br />
patients (two knees) received NSAIDs. One <strong>of</strong> these patients also received arthrocentesis. One patient was<br />
treated with arthroscopy. <strong>The</strong> remaining patients with adverse events localized to the knee received no<br />
treatment or only analgesics.<br />
Postmarket Experience: <strong>The</strong> most common adverse events reported have been pain, swelling and/or<br />
effusion in the injected knee. In some cases the effusion was considerable and caused pronounced pain.<br />
In some instances, patients have presented with knees that were tender, warm and red. It is important to<br />
rule out infection or crystalline arthropathies in such cases. Synovial fluid aspirates <strong>of</strong> varying volumes<br />
have revealed a range <strong>of</strong> cell counts, from very few to over 50,000 cells/mm 3 . Reported treatments<br />
included symptomatic therapy (e.g., rest, ice, heat, elevation, simple analgesics and NSAIDs) and/or<br />
arthrocentesis. Intra-articular corticosteroids have been used when infection was excluded. Rarely,<br />
arthroscopy has been performed. <strong>The</strong> occurrence <strong>of</strong> post-injection effusion may be associated with<br />
patient history <strong>of</strong> effusion, advanced stage <strong>of</strong> disease and/or the number <strong>of</strong> injections a patient receives.<br />
Reactions generally abate within a few days. Clinical benefit from the treatment may still occur after<br />
such reactions.<br />
<strong>The</strong> clinical trials described above included 38 patients who received a second course <strong>of</strong> Synvisc<br />
injections (132 injections). <strong>The</strong>re were twelve reports in nine patients (9.1% <strong>of</strong> injections, 23.7% <strong>of</strong><br />
patients) <strong>of</strong> knee pain and/or swelling after these injections. Reports <strong>of</strong> two additional clinical trials in<br />
which patients received repeated courses <strong>of</strong> Synvisc treatment have appeared during the post-marketing<br />
period. One <strong>of</strong> these trials included 48 patients who received 210 injections during a second course <strong>of</strong><br />
Synvisc treatment 1 ; the other contained 71 patients who received 211 injections during a second course<br />
<strong>of</strong> Synvisc treatment. A total <strong>of</strong> 157 patients have received 553 injections in the three clinical trials <strong>of</strong><br />
repeated courses <strong>of</strong> Synvisc treatment. <strong>The</strong> reports in these trials describe a total <strong>of</strong> 48 reports <strong>of</strong> adverse<br />
events localized to the injected knee in 35 patients that occurred after injections that patients had received<br />
during their second course <strong>of</strong> treatment. <strong>The</strong>se adverse events accounted for 6.3% <strong>of</strong> injections in 22.3%<br />
A division <strong>of</strong> Genzyme Corporation<br />
55 Cambridge Parkway<br />
Cambridge, MA 02142<br />
1-888-3SYNVISC<br />
www.synvisc.com<br />
<strong>of</strong> patients as compared to 2.2% <strong>of</strong> injections in 7.2% <strong>of</strong> patients in a single course <strong>of</strong> Synvisc injections.<br />
In addition, reports <strong>of</strong> two retrospective studies during the post-marketing period have described adverse<br />
events localized to the injected knee that have occurred after 4.4% and 8.5% <strong>of</strong> injections that patients had<br />
received during one or more repeated courses <strong>of</strong> Synvisc treatment. 2,3 Intra-articular infections did not<br />
occur in any <strong>of</strong> the clinical trials and have been reported only rarely during clinical use <strong>of</strong> Synvisc.<br />
OTHER ADVERSE EVENTS<br />
Clinical Trials: In three concurrently controlled clinical trials with a total <strong>of</strong> 112 patients who received<br />
Synvisc and 110 patients who received either saline or arthrocentesis, there were no statistically<br />
significant differences in the numbers or types <strong>of</strong> adverse events between the group <strong>of</strong> patients that<br />
received Synvisc and the group that received control treatments.<br />
Systemic adverse events each occurred in 10 (2.0%) <strong>of</strong> the Synvisc-treated patients. <strong>The</strong>re was one case<br />
each <strong>of</strong> rash (thorax and back) and itching <strong>of</strong> the skin following Synvisc injections in these studies. <strong>The</strong>se<br />
symptoms did not recur when these patients received additional Synvisc injections. <strong>The</strong> remaining<br />
generalized adverse events reported were calf cramps, hemorrhoid problems, ankle edema, muscle pain,<br />
tonsillitis with nausea, tachyarrhythmia, phlebitis with varicosities and low back sprain.<br />
Postmarket Experience: Other adverse events reported include: rash, hives, itching, fever, nausea,<br />
headache, dizziness, chills, muscle cramps, paresthesia, peripheral edema, malaise, respiratory difficulties,<br />
flushing and facial swelling. <strong>The</strong>re have been rare reports <strong>of</strong> thrombocytopenia coincident with Synvisc<br />
injection. <strong>The</strong>se medical events occurred under circumstances where causal relationship to Synvisc is<br />
uncertain. (Adverse events reported only in worldwide postmarketing experience, not seen in clinical trials,<br />
are considered more rare and are italicized.)<br />
DETAILED DEVICE DESCRIPTION<br />
Each syringe <strong>of</strong> Synvisc contains:<br />
Hylan polymers (hylan A + hylan B) ..........................................16 mg<br />
Sodium chloride ........................................................................17 mg<br />
Disodium hydrogen phosphate ................................................0.32 mg<br />
Sodium dihydrogen phosphate monohydrate ..........................0.08 mg<br />
Water for injection ....................................................................q.s. to 2.0 mL<br />
HOW SUPPLIED<br />
Synvisc is supplied in a 2.25 mL glass syringe containing 2 mL Synvisc.<br />
Product Number: 58468-0090-1 3 disposable syringes<br />
<strong>The</strong> contents <strong>of</strong> the syringe are sterile and nonpyrogenic.<br />
DIRECTIONS FOR USE<br />
Synvisc is administered by intra-articular injection once a week (one week apart) for a<br />
total <strong>of</strong> three injections.<br />
Precaution: Do not use Synvisc if the package has been opened or damaged. Store in original packaging<br />
(protected from light) at room temperature below 86°F (30°C). DO NOT FREEZE.<br />
Precaution: Strict aseptic administration technique must be followed.<br />
Precaution: Do not concomitantly use disinfectants containing quaternary ammonium salts for skin<br />
preparation because hyaluronan can precipitate in their presence.<br />
Precaution: Remove synovial fluid or effusion before each Synvisc injection.<br />
Do not use the same syringe for removing synovial fluid and for injecting Synvisc, but the same needle<br />
should be used.<br />
Take particular care to remove the tip cap <strong>of</strong> the syringe and needle aseptically.<br />
Twist the gray tip cap before pulling it <strong>of</strong>f, as this will minimize product leakage.<br />
Inject Synvisc into the knee joint through an 18 to 22 gauge needle.<br />
To ensure a tight seal and prevent leakage during administration, secure the needle tightly while firmly<br />
holding the luer hub.<br />
Precaution: Do not over tighten or apply excessive leverage when attaching the needle or removing the<br />
needle guard, as this may break the tip <strong>of</strong> the syringe.<br />
Do not inject anesthetics or any other medications intra-articularly into the knee while administering<br />
Synvisc therapy. This may dilute Synvisc and affect its safety and effectiveness.<br />
Precaution: <strong>The</strong> syringe containing Synvisc is intended for single use. <strong>The</strong> contents <strong>of</strong> the syringe must<br />
be used immediately after the syringe has been removed from its packaging. Inject the full 2 mL in<br />
one knee only. If treatment is bilateral, a separate syringe must be used for each knee. Discard any<br />
unused Synvisc.<br />
This brief summary is based upon the current circular, 70230602, revised November 15, 2004.<br />
References: 1. Raynauld JP, Bellamy N, Goldsmith CH, Tugwell P, Torrance GW, Pericak D, et al. (2002).<br />
An evaluation <strong>of</strong> the safety and effectiveness <strong>of</strong> repeat courses <strong>of</strong> hylan G-F 20 for treating patients with<br />
knee osteoarthritis. Osteoarthritis Research Society International, 2002 OARSI World Congress on<br />
Osteoarthritis, Sydney, Australia [Paper reference #PS128]. Presentation on File. 2. Leopold SS, Warme WJ,<br />
Pettis PD and Shott S. (2002). Increased frequency <strong>of</strong> acute local reaction to intra-articular Hylan G-F 20<br />
(Synvisc) in patients receiving more than one course <strong>of</strong> treatment. J <strong>Bone</strong> <strong>Joint</strong> Surg. 2002;84-A(9):<br />
1619-1623. 3. Waddell DD, Estey DJ and Bricker D. (2001). Retrospective tolerance <strong>of</strong> Hylan G-F 20<br />
using fluoroscopically-confirmed injection and effectiveness <strong>of</strong> retreatment in knee osteoarthritis.<br />
Proceedings <strong>of</strong> the American College <strong>of</strong> Rheumatology Annual Meeting 2001. Presentation on File.<br />
SYNVISC and GENZYME are registered trademarks <strong>of</strong> Genzyme Corporation.<br />
©2007 Genzyme Corporation. All rights reserved. Printed inUSA. S-00269.A 01/2007<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
What factors affect ingrowth?<br />
the optimal<br />
combination<br />
Does porosity equal performance?<br />
Titanium vs. Tantalum?<br />
for fixation<br />
Bonus <br />
Demineralized For answers <strong>Bone</strong> to these Matrixquestions visit www.biomet.com/porousmetalfacts<br />
or contact your Biomet sales representative.<br />
Regenerex <br />
Porous Titanium Construct<br />
Clinically proven material.<br />
Advanced porous technology.<br />
Regenerex is a trademark <strong>of</strong> Biomet Manufacturing Corp.<br />
DrivenByEngineering<br />
800.348.9500 ext. 1501 • ©2007 Biomet Orthopedics, Inc. All Rights Reserved • www.biomet.com<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
Downloaded From: http://jbjs.org/ on 01/27/2014
Now, viewing subspecialty<br />
procedures is as easy as this.<br />
Wouldn’t it be great if you could call up video procedures for<br />
your particular subspecialty at the touch <strong>of</strong> a few buttons?<br />
<strong>The</strong> Video <strong>Journal</strong> <strong>of</strong> Orthopaedics (VJO) and JBJS have greatly<br />
expanded the video <strong>of</strong>ferings at jbjs.org. In addition to the<br />
Video <strong>of</strong> the Month, a new, FREE streaming video will be<br />
introduced each Tuesday covering a different specialty. Each<br />
new video will be available for five weeks—as new ones are<br />
added, older ones will be removed. Videos can be accessed in<br />
our new online library which is organized by specialty so you<br />
can quickly find only the procedures you need.<br />
BUILD YOUR OWN CUSTOM<br />
SUBSPECIALTY COLLECTION!<br />
ORDER FOUR VJO TITLES AND<br />
A VIDEO IPOD—JUST $499!<br />
View our catalog online:<br />
www.vjortho.com<br />
To see even more procedures related to your subspecialty, VJO<br />
is now <strong>of</strong>fering you the opportunity to order customized DVDs.<br />
Looking for hand/wrist procedures? Choose any five from<br />
VJO’s extensive library and have them burned onto DVDs<br />
just for you. <strong>The</strong>se broadcast-quality videos cannot be<br />
found anywhere else. All videos are chapterized for quick<br />
and efficient viewing and include critical steps with clear<br />
demonstrations shot from multiple angles.<br />
You don’t have time to sift through volumes <strong>of</strong> material just to find<br />
the content <strong>of</strong> interest to you. With VJO and JBJS, it’s now<br />
easier to find and view the best video procedures anywhere.<br />
Video <strong>Journal</strong> <strong>of</strong> Orthopaedics<br />
805.962.3410 | www.vjortho.com<br />
www.jbjs.org<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
Downloaded From: http://jbjs.org/ on 01/27/2014
In hip-replacement surgery*...<br />
Break Down the Risk<br />
NAFT † proves FRAGMIN ® is more effective than warfarin<br />
in early postop ‡ dosing 1<br />
• 72% relative risk reduction in proximal DVT with FRAGMIN (0.8%)<br />
vs warfarin (3.0%; P=0.03) §1<br />
• 45% relative risk reduction in total DVT with FRAGMIN (13.1%)<br />
vs warfarin (24.0%; P
FRAGMIN<br />
(dalteparin sodium injection)<br />
For Subcutaneous Use Only<br />
BRIEF SUMMARY: For full prescribing information, see package insert.<br />
SPINAL/EPIDURAL HEMATOMAS<br />
When neuraxial anesthesia (epidural/spinal anesthesia) or spinal puncture is employed, patients anticoagulated or<br />
scheduled to be anticoagulated with low molecular weight heparins or heparinoids for prevention <strong>of</strong><br />
thromboembolic complications are at risk <strong>of</strong> developing an epidural or spinal hematoma which can result in<br />
long-term or permanent paralysis. <strong>The</strong> risk <strong>of</strong> these events is increased by the use <strong>of</strong> indwelling epidural catheters<br />
for administration <strong>of</strong> analgesia or by the concomitant use <strong>of</strong> drugs affecting hemostasis such as non steroidal<br />
anti-inflammatory drugs (NSAIDs), platelet inhibitors, or other anticoagulants. <strong>The</strong> risk also appears to be<br />
increased by traumatic or repeated epidural or spinal puncture. Patients should be frequently monitored for signs<br />
and symptoms <strong>of</strong> neurological impairment. If neurological compromise is noted, urgent treatment is necessary.<br />
<strong>The</strong> physician should consider the potential benefit versus risk before neuraxial intervention in patients<br />
anticoagulated or to be anticoagulated for thromboprophylaxis (also see WARNINGS, Hemorrhage and<br />
PRECAUTIONS, Drug Interactions).<br />
INDICATIONS AND USAGE<br />
FRAGMIN Injection is indicated for the prophylaxis <strong>of</strong> ischemic complications in unstable angina and non-Q-wave<br />
myocardial infarction, when concurrently administered with aspirin therapy (as described in CLINICAL TRIALS,<br />
Prophylaxis <strong>of</strong> Ischemic Complications in Unstable Angina and Non-Q-Wave Myocardial Infarction).<br />
FRAGMIN is also indicated for the prophylaxis <strong>of</strong> deep vein thrombosis (DVT), which may lead to pulmonary<br />
embolism (PE):<br />
• In patients undergoing hip replacement surgery;<br />
• In patients undergoing abdominal surgery who are at risk for thromboembolic complications;<br />
• In medical patients who are at risk for thromboembolic complications due to severely restricted mobility<br />
during acute illness.<br />
FRAGMIN is also indicated for the extended treatment <strong>of</strong> symptomatic venous thromboembolism (VTE) (proximal<br />
DVT and/or PE), to reduce the recurrence <strong>of</strong> VTE in patients with cancer.<br />
CONTRAINDICATIONS<br />
FRAGMIN Injection is contraindicated in patients with known hypersensitivity to the drug, active major bleeding, or<br />
thrombocytopenia associated with positive in vitro tests for antiplatelet antibody in the presence <strong>of</strong> FRAGMIN.<br />
Patients undergoing regional anesthesia should not receive FRAGMIN for unstable angina or non-Q-wave myocardial<br />
infarction, and patients with cancer undergoing regional anesthesia should not receive FRAGMIN for extended<br />
treatment <strong>of</strong> symptomatic VTE, due to an increased risk <strong>of</strong> bleeding associated with the dosage <strong>of</strong> FRAGMIN<br />
recommended for these indications. Patients with known hypersensitivity to heparin or pork products should not be<br />
treated with FRAGMIN.<br />
WARNINGS<br />
FRAGMIN Injection is not intended for intramuscular administration. FRAGMIN cannot be used interchangeably (unit<br />
for unit) with unfractionated heparin or other low molecular weight heparins. FRAGMIN should be used with<br />
extreme caution in patients with history <strong>of</strong> heparin-induced thrombocytopenia.<br />
Hemorrhage: FRAGMIN, like other anticoagulants, should be used with extreme caution in patients who have an<br />
increased risk <strong>of</strong> hemorrhage, such as those with severe uncontrolled hypertension, bacterial endocarditis,<br />
congenital or acquired bleeding disorders, active ulceration and angiodysplastic gastrointestinal disease,<br />
hemorrhagic stroke, or shortly after brain, spinal or ophthalmological surgery. Spinal or epidural hematomas can<br />
occur with the associated use <strong>of</strong> low molecular weight heparins or heparinoids and neuraxial (spinal/epidural)<br />
anesthesia or spinal puncture, which can result in long-term or permanent paralysis. <strong>The</strong> risk <strong>of</strong> these events<br />
is higher with the use <strong>of</strong> indwelling epidural catheters or concomitant use <strong>of</strong> additional drugs affecting<br />
hemostasis such as NSAIDs (see boxed WARNING and ADVERSE REACTIONS, Ongoing Safety Surveillance).<br />
As with other anticoagulants, bleeding can occur at any site during therapy with FRAGMIN. An unexpected drop in<br />
hematocrit or blood pressure should lead to a search for a bleeding site.<br />
Thrombocytopenia: In FRAGMIN clinical trials supporting non-cancer indications, platelet counts <strong>of</strong><br />
Three <strong>of</strong> the major bleeding events that occurred by Day 21 were fatal, all due to gastrointestinal hemorrhage<br />
(two patients in the group treated with FRAGMIN and one in the group receiving placebo). Two deaths<br />
occurred after Day 21: one patient in the placebo group died from a subarachnoid hemorrhage that started on<br />
Day 55, and one patient died on day 71 (two months after receiving the last dose <strong>of</strong> FRAGMIN) from a<br />
subdural hematoma.<br />
Patients with Cancer and Acute Symptomatic Venous Thromboembolism<br />
Table 12 summarizes the number <strong>of</strong> patients with bleeding events that occurred in the clinical trial <strong>of</strong> patients with<br />
cancer and acute symptomatic venous thromboembolism. A bleeding event was considered major if it: 1) was<br />
accompanied by a decrease in hemoglobin <strong>of</strong> >2 g/dL in connection with clinical symptoms; 2) occurred at a critical<br />
site (intraocular, spinal/epidural, intracranial, retroperitoneal, or pericardial bleeding); 3) required transfusion <strong>of</strong><br />
>2 units <strong>of</strong> blood products; or 4) led to death. Minor bleeding was classified as clinically overt bleeding that did not<br />
meet criteria for major bleeding. At the end <strong>of</strong> the six-month study, a total <strong>of</strong> 46 (13.6%) patients in the FRAGMIN<br />
arm and 62 (18.5%) patients in the OAC arm experienced any bleeding event. One bleeding event (hemoptysis in a<br />
patient in the FRAGMIN arm at Day 71) was fatal.<br />
Table 12: Bleeding Events (Major and Any) (As treated population) 1<br />
Study period FRAGMIN OAC<br />
200 IU/kg (max. 18,000 IU) s.c. FRAGMIN 200 IU/kg (max 18,000 IU) s.c.<br />
once daily x 1 month, then 150 IU/kg once daily x 5-7 days and OAC<br />
(max 18,000 IU) s.c. once daily x 5 months for 6 months (target INR 2-3)<br />
Number Patients with Patients with Number Patients with Patients with<br />
at risk Major Bleeding Any Bleeding at Risk Major Bleeding Any Bleeding<br />
n(%) n(%) n(%) n(%)<br />
Total during study 338 19 (5.6) 46 (13.6) 335 12 (3.6) 62 (18.5)<br />
Week 1 338 4 (1.2) 15 (4.4) 335 4 (1.2) 12 (3.6)<br />
Weeks 2-4 332 9 (2.7) 17 (5.1) 321 1 (0.3) 12 (3.7)<br />
Weeks 5-28 297 9 (3.0) 26 (8.8) 267 8 (3.0) 40 (15.0)<br />
1Patients with multiple bleeding episodes within any time interval were counted only once in that interval. However,<br />
patients with multiple bleeding episodes that occurred at different time intervals were counted once in each interval<br />
in which the event occurred.<br />
Thrombocytopenia: See WARNINGS: Thrombocytopenia.<br />
Other: Allergic Reactions: Allergic reactions (i.e., pruritus, rash, fever, injection site reaction, bulleous eruption)<br />
have occurred rarely. A few cases <strong>of</strong> anaphylactoid reactions have been reported. Local Reactions: Pain at the<br />
injection site, the only non-bleeding event determined to be possibly or probably related to treatment with FRAGMIN<br />
and reported at a rate <strong>of</strong> at least 2% in the group treated with FRAGMIN, was reported in 4.5% <strong>of</strong> patients treated<br />
with FRAGMIN 5000 IU once daily vs 11.8% <strong>of</strong> patients treated with heparin 5000 U twice daily in the abdominal<br />
surgery trials. In the hip replacement trials, pain at injection site was reported in 12% <strong>of</strong> patients treated with<br />
FRAGMIN 5000 IU once daily vs 13% <strong>of</strong> patients treated with heparin 5000 U three times a day.<br />
Ongoing Safety Surveillance: Since first international market introduction in 1985, there have been more than<br />
15 reports <strong>of</strong> epidural or spinal hematoma formation with concurrent use <strong>of</strong> dalteparin sodium and spinal/epidural<br />
anesthesia or spinal puncture. <strong>The</strong> majority <strong>of</strong> patients had postoperative indwelling epidural catheters placed for<br />
analgesia or received additional drugs affecting hemostasis. In some cases the hematoma resulted in long-term or<br />
permanent paralysis (partial or complete). Because these events were reported voluntarily from a population <strong>of</strong><br />
unknown size, estimates <strong>of</strong> frequency cannot be made.<br />
Post-Marketing Experience: Skin necrosis has occurred rarely. <strong>The</strong>re have been isolated cases <strong>of</strong> alopecia reported<br />
that improved on drug discontinuation.<br />
OVERDOSAGE<br />
Symptoms/Treatment: An excessive dosage <strong>of</strong> FRAGMIN Injection may lead to hemorrhagic complications. <strong>The</strong>se<br />
may generally be stopped by the slow intravenous injection <strong>of</strong> protamine sulfate (1% solution), at a dose <strong>of</strong> 1 mg<br />
protamine for every 100 anti-Xa IU <strong>of</strong> FRAGMIN given. A second infusion <strong>of</strong> 0.5 mg protamine sulfate per 100<br />
anti-Xa IU <strong>of</strong> FRAGMIN may be administered if the APTT measured 2 to 4 hours after the first infusion remains<br />
prolonged. Even with these additional doses <strong>of</strong> protamine, the APTT may remain more prolonged than would usually<br />
be found following administration <strong>of</strong> conventional heparin. In all cases, the anti-Factor Xa activity is never completely<br />
neutralized (maximum about 60 to 75%). Particular care should be taken to avoid overdosage with protamine sulfate.<br />
Administration <strong>of</strong> protamine sulfate can cause severe hypotensive and anaphylactoid reactions. Because fatal<br />
reactions, <strong>of</strong>ten resembling anaphylaxis, have been reported with protamine sulfate, it should be given only when<br />
resuscitation techniques and treatment <strong>of</strong> anaphylactic shock are readily available. For additional information, consult<br />
the labeling <strong>of</strong> Protamine Sulfate Injection, USP, products. A single subcutaneous dose <strong>of</strong> 100,000 IU/kg <strong>of</strong> FRAGMIN<br />
to mice caused a mortality <strong>of</strong> 8% (1/12) whereas 50,000 IU/kg was a non-lethal dose. <strong>The</strong> observed sign was<br />
hematoma at the site <strong>of</strong> injection.<br />
Rx only<br />
U.S. Patent 4,303,651<br />
April 2007 FR080231 © 2007 Pfizer Inc.<br />
818 312 112 All rights reserved. Printed in USA/July 2007<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
Adv 64<br />
JBJS [Br] Abstracts Now Available<br />
Trauma<br />
Fractures <strong>of</strong> the distal third <strong>of</strong> the humerus with palsy <strong>of</strong> the radial<br />
nerve<br />
MANAGEMENT USING MINIMALLY-INVASIVE<br />
PERCUTANEOUS PLATE OSTEOSYNTHESIS<br />
A. Livani, W. D. Belangero, and R. Castro de Medeiros<br />
From the State University <strong>of</strong> Campinas, Sao Paulo, Brazil<br />
J <strong>Bone</strong> <strong>Joint</strong> Surg (Br) 2006;88-B:1625-28<br />
Fractures <strong>of</strong> the distal third <strong>of</strong> the humerus may be complicated<br />
by complete lesions <strong>of</strong> the radial nerve which may be<br />
entrapped or compressed by bone fragments. Indirect reduction<br />
and internal fixation may result in a permanent nerve lesion.<br />
We describe the treatment <strong>of</strong> these lesions by insertion <strong>of</strong><br />
a bridge plate using the minimally-invasive percutaneous technique.<br />
Six patients were operated on and showed complete<br />
functional recovery. Healing <strong>of</strong> the fractures occurred at a mean<br />
<strong>of</strong> 2.7 months (2 to 3) and complete neurological recovery by a<br />
mean <strong>of</strong> 2.3 months (1 to 5). In one patient infection occurred<br />
which resolved after removal <strong>of</strong> the implant.<br />
gen 1 (sca-1+) and stem cell factor receptor, CD117 (c-kit+)<br />
in order to identify the endothelial precursor cell population.<br />
Immunomagnetically-enriched sca-1+ mononuclear cell<br />
(MNCsca-1+) populations were then cultured and examined<br />
for functional vascular endothelial differentiation. <strong>Bone</strong> marrow<br />
MNCsca-1+,c-kit+ counts increased almost tw<strong>of</strong>old within<br />
48 hours <strong>of</strong> the event, compared with baseline levels, before<br />
decreasing by 72 hours.<br />
Sca-1+ mononuclear cell populations in culture from<br />
samples <strong>of</strong> bone marrow at 48 hours bound together Ulex<br />
Europus-1, and incorporated fluorescent 1,1'-dioctadecyl-<br />
3,3,3,’3'-tetramethylindocarbocyanine perchlorate-labelled<br />
acetylated low-density lipoprotein intracellularily, both characteristics<br />
<strong>of</strong> mature endothelium.<br />
Our findings suggest that a systemic provascular response <strong>of</strong><br />
bone marrow is initiated by musculoskeletal trauma. Its therapeutic<br />
manipulation may have implications for the potential<br />
enhancement <strong>of</strong> neovascularisation and the healing <strong>of</strong> fractures.<br />
Upper Limb<br />
Research<br />
A systemic provascular response in bone marrow to musculoskeletal<br />
trauma in mice<br />
A. J. Laing, J. P. Dillon, E.T. Condon, J. C. C<strong>of</strong>fey, J. T. Street, J. H.<br />
Wang, A. J. McGuinness, and H. P. Redmond<br />
J <strong>Bone</strong> <strong>Joint</strong> Surg Br 2007 89-B: 116-120<br />
Post-natal vasculogenesis, the process by which vascular<br />
committed bone marrow stem cells or endothelial precursor<br />
cells migrate, differentiate and incorporate into the nacent<br />
endothelium and thereby contribute to physiological and pathological<br />
neurovascularisation, has stimulated much interest. Its<br />
contribution to neovascularisation <strong>of</strong> tumours, wound healing<br />
and revascularisation associated with ischaemia <strong>of</strong> skeletal and<br />
cardiac muscles is well established. We evaluated the responses<br />
<strong>of</strong> endothelial precursor cells in bone marrow to musculoskeletal<br />
trauma in mice.<br />
<strong>Bone</strong> marrow from six C57 Black 6 mice subjected to a<br />
standardised, closed fracture <strong>of</strong> the femur, was analysed for<br />
the combined expression <strong>of</strong> cell-surface markers stem cell anti-<br />
<strong>The</strong> outcome <strong>of</strong> peri-operative humeral condylar fractures after<br />
total elbow replacement in patients with rheumatoid arthritis<br />
H. Ito, T. Matsumoto, H. Yoshitomi, R. Kakinoki, and T. Nakamura<br />
J <strong>Bone</strong> <strong>Joint</strong> Surg Br 2007 89-B: 62-65<br />
We compared the outcome <strong>of</strong> peri-operative humeral condylar<br />
fractures in patients undergoing a Coonrad-Morrey semiconstrained<br />
total elbow replacement with that <strong>of</strong> patients with<br />
rheumatoid arthritis undergoing the same procedure without<br />
fractures. In a consecutive series <strong>of</strong> 40 elbows in 33 patients,<br />
13 elbows had a fracture in either condyle peri-operatively, and<br />
27 elbows were intact. <strong>The</strong> fractured condyle was either fixed<br />
internally or excised. We found no statistical difference in the<br />
patients’ background, such as age, length <strong>of</strong> follow-up, immobilisation<br />
period, Larsen’s radiological grade, or Steinbrocker’s<br />
stage and functional class. <strong>The</strong>re was also no statistical difference<br />
between the groups in relation to the Mayo Elbow Performance<br />
Score, muscle strength, range <strong>of</strong> movement, or radiolucency<br />
around the implants at a mean <strong>of</strong> 4.8 years (1.1 to 8.0) followup.<br />
We conclude that fractured condyles can be successfully<br />
treated with either internal fixation or excision, and cause no<br />
harmful effect.<br />
Downloaded From: http://jbjs.org/ on 01/27/2014<br />
THE JOURNAL OF<br />
BONE AND JOINT SURGERY<br />
www.jbjs.org.uk
Simplex <strong>Bone</strong> Cements<br />
Because the structure is only<br />
as good as the foundation<br />
For more information on Simplex <strong>Bone</strong> Cements, contact your Stryker<br />
Orthopaedics Representative at 866-447-3627 or visit www.stryker.com<br />
Simplex P, Simplex P with Tobramycin, Simplex P SpeedSet<br />
Stryker Orthopaedics 325 Corporate Drive, Mahwah, NJ 07430<br />
A surgeon is the best person to decide with the patient which treatments and products are right for them.<br />
Surgeons must always rely on their own clinical judgement when deciding which treatments and procedure<br />
to use with patients. Copyright © 2007 Stryker. Stryker Corporation or its divisions or other corporate<br />
affiliated entities own, use or have applied for the following trademarks: Stryker, Simplex SpeedSet.<br />
A C DF G B E E B G FD C<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
moving beyond simple wear reduction<br />
Only E-Poly HXLPE uses vitamin E to provide high strength and true oxidative stability.<br />
Not X3 ®<br />
Not Longevity ®<br />
Not Marathon ®<br />
only E-Poly <br />
E-Poly is a trademark <strong>of</strong> Biomet Manufacturing Corp.<br />
X3® is a trademark <strong>of</strong> Howmedica Osteonics Corp.<br />
Longevity® is a trademark <strong>of</strong> Zimmer Inc.<br />
Marathon® is a trademark <strong>of</strong> Johnson and Johnson Corp.<br />
Biologics • Bracing • Micr<strong>of</strong>i xation • Orthopedics • Osteobiologics • Spine • Sports Medicine • Trauma • 3i<br />
biomet.com • 800.348.9500 x 1501<br />
©2007 Biomet Orthopedics, Inc.<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
Adv <br />
Advertisers<br />
september 2007<br />
3M .........................................................Adv 23<br />
800.228.3957<br />
Genzyme .........................................Adv 55,56<br />
www.synvisc.com<br />
888.3SYNVISC<br />
Nexa Orthopaedics .............................Adv 68<br />
www.nexaortho.com<br />
800.835.8480<br />
A.A.O.S. .................................................Adv 30<br />
www.aaos.org<br />
800.626.6726<br />
Hand Biomechanics Lab ......................Adv 6<br />
www.handbiolab.com<br />
888.Wristjack<br />
O.R.E.F. ..................................................Adv 34<br />
www.oref.org<br />
800.384.4356<br />
Acumed ....................................Adv 25,41,52<br />
800.627.9957<br />
AOA ........................................................Adv 18<br />
www.aoassn.org<br />
847.318.7330<br />
Arthrex .....................................................Adv 2<br />
www.arthrex.com<br />
800.934.9957<br />
Innomed ..................................................Adv 8<br />
www.innomed.net<br />
800.548.2362<br />
Innovative Medical Products .............Adv 24<br />
www.impmedical.com<br />
800.467.4944<br />
Integra ...................................................Adv 21<br />
www.ilstraining.com<br />
800.654.2873<br />
Orthopaedics Overseas ......................Adv 46<br />
www.hvousa.org<br />
202.296.0928<br />
Regeneration Technologies ...............Adv 28<br />
www.rtitechnology.com<br />
877.343.6832<br />
Smith & Nephew Inc. ..........Adv 4,10,36,42<br />
www.smith-nephew.com<br />
Arthrosurface .......................................Adv 47<br />
www.arthrosurface.com<br />
508.520.3003<br />
Baxter Biosurgery ..........................Adv 53,54<br />
www.baxterbiosurgery.com<br />
847.940.5692<br />
Biomet .......................................Adv 27,57,66<br />
www.biomet.com<br />
219.267.6639<br />
DePuy ....................................................Adv 49<br />
www.depuy.com<br />
Eisai Pharmaceuticals ............Adv 61,62,63<br />
www.fragmin.com<br />
Excel Medical Solutions .....................Adv 50<br />
www.bonefoam.com<br />
612.338.1400<br />
Jurgan Development & Mfg. .............Adv 43<br />
800.587.4262<br />
KCI .........................................................Adv 12<br />
www.kci1.com<br />
888.275.4524<br />
King Pharmaceuticals ............Adv 38,39,40<br />
www.kingpharm.com<br />
KFx Medical ........................................Cover III<br />
www.kfxmedical.com<br />
866.883.8718<br />
Lima-Lto spa ........................................Adv 44<br />
www.lima.it<br />
+39 043 29 455<br />
Medtronic S<strong>of</strong>amor Danek .............................<br />
Adv 45,46,124,Cover IV<br />
www.back.com<br />
866.4.INFUSE<br />
Stryker Orthopaedics ......................................<br />
Adv 29,31,33,37,51,65<br />
www.stryker.com<br />
Union Surgical .....................................Adv 54<br />
www.unionsurgical.com<br />
215.925.5208<br />
Video <strong>Journal</strong> <strong>of</strong> Orthopaedics ....Adv 58,59<br />
www.vjortho.com<br />
805.962.3410<br />
W.Link GmbH & Co. ............................Adv 35<br />
www.linkorthopaedics.com<br />
Zimmer .................................................Adv 19<br />
www.zimmer.com<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
Downloaded From: http://jbjs.org/ on 01/27/2014
INTRODUCING<br />
STRONG<br />
SIMPLE<br />
VERSATILE<br />
Double Row Rotator Cuff Repair<br />
• No knot tying<br />
• No suture passing<br />
To locate a KFx Medical Sales Representative call toll free, 1.866.883.8718 www.kfxmedical.com<br />
Downloaded From: http://jbjs.org/ on 01/27/2014
See brief summary on page 110<br />
Downloaded From: http://jbjs.org/ on 01/27/2014