New Acetonyl Palladium(II) Complexes - Universidad de Murcia
New Acetonyl Palladium(II) Complexes - Universidad de Murcia
New Acetonyl Palladium(II) Complexes - Universidad de Murcia
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>New</strong> <strong>Acetonyl</strong> <strong>Palladium</strong>(<strong>II</strong>) <strong>Complexes</strong> Organometallics, Vol. 27, No. 15, 2008 3983<br />
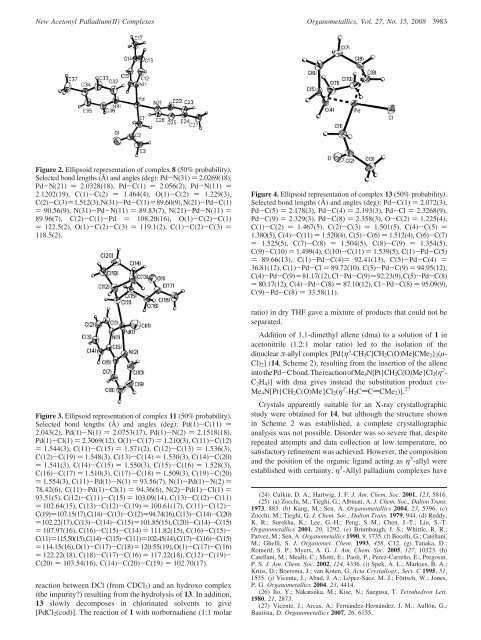
Figure 2. Ellipsoid representation of complex 8 (50% probability).<br />
Selected bond lengths (Å) and angles (<strong>de</strong>g): Pd-N(31) ) 2.0269(18),<br />
Pd-N(21) ) 2.0328(18), Pd-C(1) ) 2.056(2), Pd-N(11) )<br />
2.1202(19), C(1)-C(2) ) 1.464(4), O(1)-C(2) ) 1.229(3),<br />
C(2)-C(3))1.512(3),N(31)-Pd-C(1))89.60(9),N(21)-Pd-C(1)<br />
) 90.56(9), N(31)-Pd-N(11) ) 89.83(7), N(21)-Pd-N(11) )<br />
89.96(7), C(2)-C(1)-Pd ) 108.20(16), O(1)-C(2)-C(1)<br />
) 122.5(2), O(1)-C(2)-C(3) ) 119.1(2), C(1)-C(2)-C(3) )<br />
118.5(2).<br />
Figure 3. Ellipsoid representation of complex 11 (50% probability).<br />
Selected bond lengths (Å) and angles (<strong>de</strong>g): Pd(1)-C(11) )<br />
2.043(2), Pd(1)-N(1) ) 2.0753(17), Pd(1)-N(2) ) 2.1518(18),<br />
Pd(1)-Cl(1) ) 2.3069(12), O(1)-C(17) ) 1.210(3), C(11)-C(12)<br />
) 1.544(3), C(11)-C(15) ) 1.571(2), C(12)-C(13) ) 1.536(3),<br />
C(12)-C(19) ) 1.548(3), C(13)-C(14) ) 1.530(3), C(14)-C(20)<br />
) 1.541(3), C(14)-C(15) ) 1.550(3), C(15)-C(16) ) 1.528(3),<br />
C(16)-C(17) ) 1.510(3), C(17)-C(18) ) 1.509(3), C(19)-C(20)<br />
) 1.554(3), C(11)-Pd(1)-N(1) ) 93.56(7), N(1)-Pd(1)-N(2) )<br />
78.42(6), C(11)-Pd(1)-Cl(1) ) 94.36(6), N(2)-Pd(1)-Cl(1) )<br />
93.51(5), C(12)-C(11)-C(15) ) 103.09(14), C(13)-C(12)-C(11)<br />
) 102.64(15), C(13)-C(12)-C(19) ) 100.61(17), C(11)-C(12)-<br />
C(19))107.15(17),C(14)-C(13)-C(12))94.74(16),C(13)-C(14)-C(20)<br />
)102.22(17),C(13)-C(14)-C(15))101.85(15),C(20)-C(14)-C(15)<br />
) 107.97(16), C(16)-C(15)-C(14) ) 111.82(15), C(16)-C(15)-<br />
C(11))115.50(15),C(14)-C(15)-C(11))102.45(14),C(17)-C(16)-C(15)<br />
)114.15(16),O(1)-C(17)-C(18))120.55(19),O(1)-C(17)-C(16)<br />
) 122.22(18), C(18)-C(17)-C(16) ) 117.22(18), C(12)-C(19)-<br />
C(20) ) 103.54(16), C(14)-C(20)-C(19) ) 102.70(17).<br />
reaction between DCl (from CDCl 3 ) and an hydroxo complex<br />
(the impurity?) resulting from the hydrolysis of 13. In addition,<br />
13 slowly <strong>de</strong>composes in chlorinated solvents to give<br />
[PdCl 2 (cod)]. The reaction of 1 with norbornadiene (1:1 molar<br />
Figure 4. Ellipsoid representation of complex 13 (50% probability).<br />
Selected bond lengths (Å) and angles (<strong>de</strong>g): Pd-C(1) ) 2.072(3),<br />
Pd-C(5) ) 2.178(3), Pd-C(4) ) 2.193(3), Pd-Cl ) 2.3268(9),<br />
Pd-C(9) ) 2.329(3), Pd-C(8) ) 2.358(3), O-C(2) ) 1.225(4),<br />
C(1)-C(2) ) 1.467(5), C(2)-C(3) ) 1.501(5), C(4)-C(5) )<br />
1.380(5), C(4)-C(11) ) 1.520(4), C(5)-C(6) ) 1.512(4), C(6)-C(7)<br />
) 1.525(5), C(7)-C(8) ) 1.504(5), C(8)-C(9) ) 1.354(5),<br />
C(9)-C(10) ) 1.499(4), C(10)-C(11) ) 1.539(5), C(1)-Pd-C(5)<br />
) 89.66(13), C(1)-Pd-C(4)) 92.41(13), C(5)-Pd-C(4) )<br />
36.81(12), C(1)-Pd-Cl ) 89.72(10), C(5)-Pd-C(9) ) 94.95(12),<br />
C(4)-Pd-C(9))81.17(12),Cl-Pd-C(9))92.23(9),C(5)-Pd-C(8)<br />
) 80.17(12), C(4)-Pd-C(8) ) 87.10(12), Cl-Pd-C(8) ) 95.09(9),<br />
C(9)-Pd-C(8) ) 33.58(11).<br />
ratio) in dry THF gave a mixture of products that could not be<br />
separated.<br />
Addition of 1,1-dimethyl allene (dma) to a solution of 1 in<br />
acetonitrile (1.2:1 molar ratio) led to the isolation of the<br />
dinuclear π-allyl complex [Pd{η 3 -CH 2 C[CH 2 C(O)Me]CMe 2 } 2 (µ-<br />
Cl) 2 ](14, Scheme 2), resulting from the insertion of the allene<br />
intothePd-Cbond.ThereactionofMe 4 N[Pt{CH 2 C(O)Me}Cl 2 (η 2 -<br />
C 2 H 4 )] with dma gives instead the substitution product cis-<br />
Me 4 N[Pt{CH 2 C(O)Me}Cl 2 (η 2 -H 2 CdCdCMe 2 )]. 27<br />
Crystals apparently suitable for an X-ray crystallographic<br />
study were obtained for 14, but although the structure shown<br />
in Scheme 2 was established, a complete crystallographic<br />
analysis was not possible. Disor<strong>de</strong>r was so severe that, <strong>de</strong>spite<br />
repeated attempts and data collection at low temperature, no<br />
satisfactory refinement was achieved. However, the composition<br />
and the position of the organic ligand acting as η 3 -allyl were<br />
established with certainty. η 3 -Allyl palladium complexes have<br />
(24) Culkin, D. A.; Hartwig, J. F. J. Am. Chem. Soc. 2001, 123, 5816.<br />
(25) (a) Zocchi, M.; Tieghi, G.; Albinati, A. J. Chem. Soc., Dalton Trans.<br />
1973, 883. (b) Kang, M.; Sen, A. Organometallics 2004, 23, 5396. (c)<br />
Zocchi, M.; Tieghi, G. J. Chem. Soc., Dalton Trans. 1979, 944. (d) Reddy,<br />
K. R.; Surekha, K.; Lee, G.-H.; Peng, S.-M.; Chen, J.-T.; Liu, S.-T.<br />
Organometallics 2001, 20, 1292. (e) Brumbaugh, J. S.; Whittle, R. R.;<br />
Parvez, M.; Sen, A. Organometallics 1990, 9, 1735. (f) Bocelli, G.; Catellani,<br />
M.; Ghelli, S. J. Organomet. Chem. 1993, 458, C12. (g) Tanaka, D.;<br />
Romeril, S. P.; Myers, A. G. J. Am. Chem. Soc. 2005, 127, 10323. (h)<br />
Catellani, M.; Mealli, C.; Motti, E.; Paoli, P.; Perez-Carreño, E.; Pregosin,<br />
P. S. J. Am. Chem. Soc. 2002, 124, 4336. (i) Spek, A. L.; Markies, B. A.;<br />
Kruis, D.; Boersma, J.; van Koten, G. Acta Crystallogr., Sect. C 1995, 51,<br />
1535. (j) Vicente, J.; Abad, J. A.; López-Sáez, M. J.; Förtsch, W.; Jones,<br />
P. G. Organometallics 2004, 23, 4414.<br />
(26) Ito, Y.; Nakatsuka, M.; Kise, N.; Saegusa, T. Tetrahedron Lett.<br />
1980, 21, 2873.<br />
(27) Vicente, J.; Arcas, A.; Fernán<strong>de</strong>z-Hernán<strong>de</strong>z, J. M.; Aullón, G.;<br />
Bautista, D. Organometallics 2007, 26, 6155.