10 Yeast Growth and the Cell Cycle
10 Yeast Growth and the Cell Cycle
10 Yeast Growth and the Cell Cycle
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>10</strong> <strong>Yeast</strong> <strong>Growth</strong> <strong>and</strong> <strong>the</strong> <strong>Cell</strong> <strong>Cycle</strong><br />
<strong>10</strong>.1 Vegetative Reproduction in <strong>Yeast</strong><br />
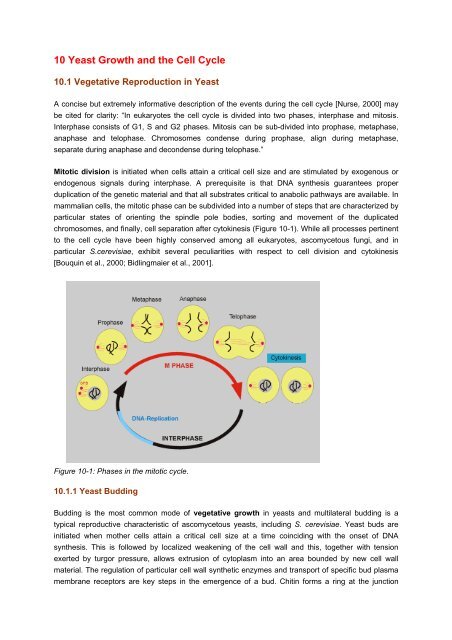
A concise but extremely informative description of <strong>the</strong> events during <strong>the</strong> cell cycle [Nurse, 2000] may<br />
be cited for clarity: “In eukaryotes <strong>the</strong> cell cycle is divided into two phases, interphase <strong>and</strong> mitosis.<br />
Interphase consists of G1, S <strong>and</strong> G2 phases. Mitosis can be sub-divided into prophase, metaphase,<br />
anaphase <strong>and</strong> telophase. Chromosomes condense during prophase, align during metaphase,<br />
separate during anaphase <strong>and</strong> decondense during telophase.”<br />
Mitotic division is initiated when cells attain a critical cell size <strong>and</strong> are stimulated by exogenous or<br />
endogenous signals during interphase. A prerequisite is that DNA syn<strong>the</strong>sis guarantees proper<br />
duplication of <strong>the</strong> genetic material <strong>and</strong> that all substrates critical to anabolic pathways are available. In<br />
mammalian cells, <strong>the</strong> mitotic phase can be subdivided into a number of steps that are characterized by<br />
particular states of orienting <strong>the</strong> spindle pole bodies, sorting <strong>and</strong> movement of <strong>the</strong> duplicated<br />
chromosomes, <strong>and</strong> finally, cell separation after cytokinesis (Figure <strong>10</strong>-1). While all processes pertinent<br />
to <strong>the</strong> cell cycle have been highly conserved among all eukaryotes, ascomycetous fungi, <strong>and</strong> in<br />
particular S.cerevisiae, exhibit several peculiarities with respect to cell division <strong>and</strong> cytokinesis<br />
[Bouquin et al., 2000; Bidlingmaier et al., 2001].<br />
Figure <strong>10</strong>-1: Phases in <strong>the</strong> mitotic cycle.<br />
<strong>10</strong>.1.1 <strong>Yeast</strong> Budding<br />
Budding is <strong>the</strong> most common mode of vegetative growth in yeasts <strong>and</strong> multilateral budding is a<br />
typical reproductive characteristic of ascomycetous yeasts, including S. cerevisiae. <strong>Yeast</strong> buds are<br />
initiated when mo<strong>the</strong>r cells attain a critical cell size at a time coinciding with <strong>the</strong> onset of DNA<br />
syn<strong>the</strong>sis. This is followed by localized weakening of <strong>the</strong> cell wall <strong>and</strong> this, toge<strong>the</strong>r with tension<br />
exerted by turgor pressure, allows extrusion of cytoplasm into an area bounded by new cell wall<br />
material. The regulation of particular cell wall syn<strong>the</strong>tic enzymes <strong>and</strong> transport of specific bud plasma<br />
membrane receptors are key steps in <strong>the</strong> emergence of a bud. Chitin forms a ring at <strong>the</strong> junction
etween <strong>the</strong> mo<strong>the</strong>r cell <strong>and</strong> <strong>the</strong> newly emerging bud to finally result in <strong>the</strong> generation of a daughter<br />
cell. After cell separation, this ring will be retained at <strong>the</strong> surface of <strong>the</strong> mo<strong>the</strong>r cell <strong>and</strong> form <strong>the</strong> socalled<br />
bud scar (Figures <strong>10</strong>-2 <strong>and</strong> <strong>10</strong>-3), <strong>and</strong> a birth scar at <strong>the</strong> surface of <strong>the</strong> daughter. The number of<br />
bud scars left on <strong>the</strong> surface of a yeast cell is a useful determinant of cellular age. Several recent<br />
papers have been devoted to budding in yeast [Roemer et al., 1996; Barral et al., 1999; Barrett et al.,<br />
2000; Manning et al., 1999; Sheu et al., 2000; Swaroop et al., 2000; Vogel et al., 2000, Ni & Snyder,<br />
2001].<br />
Figure <strong>10</strong>-2: Budding yeast cell.<br />
Figure <strong>10</strong>-3: <strong>Yeast</strong> bud <strong>and</strong> bud scar.<br />
During bud formation, only <strong>the</strong> bud but not <strong>the</strong> mo<strong>the</strong>r cell will grow. Once mitosis is complete <strong>and</strong> <strong>the</strong><br />
bud nucleus <strong>and</strong> o<strong>the</strong>r organelles have migrated into <strong>the</strong> bud, cytokinesis commences <strong>and</strong> a septum is<br />
formed in <strong>the</strong> isthmus between mo<strong>the</strong>r <strong>and</strong> daughter. A ring of proteins, called septins (Figure <strong>10</strong>-4),<br />
are involved in positioning cell division in that <strong>the</strong>y define <strong>the</strong> cleavage plain which bisects <strong>the</strong> spindle<br />
axis at cytokinesis. These septins encircle <strong>the</strong> neck between mo<strong>the</strong>r <strong>and</strong> daughter for <strong>the</strong> duration of<br />
<strong>the</strong> cell cycle.<br />
In S. cerevisiae, cell size at division is asymmetrical with buds being smaller than mo<strong>the</strong>r cells when<br />
<strong>the</strong>y separate. Also cell division cycle times are different, because daughter cells need time (in G1<br />
phase) to attain <strong>the</strong> critical cell size before <strong>the</strong>y are prepared to bud.
Table <strong>10</strong>-1: Examples of components important for budding<br />
Genes Gene product characteristics Mutant phenotypes<br />
General bud-site selection<br />
R<strong>and</strong>om budding in haploids <strong>and</strong> diploids<br />
BUD1/RSR1<br />
Ras-related protein<br />
BUD2<br />
GTPase-activating protein (GAP)<br />
BUD5<br />
GDP-GTP exchange factor (GEF)<br />
Axial bud-site selection<br />
Bipolar budding in haploids<br />
BUD3<br />
Novel<br />
BUD4<br />
GTP_binding domain<br />
AKL1<br />
a-factor protease<br />
BUD<strong>10</strong>/AXL2<br />
Type-I plasma membrane glycoprotein<br />
Polarity establishment<br />
Round, multinucleate cells unable to bud<br />
CDC24<br />
GEF for Cdc42p<br />
CDC42<br />
Rho/Rac GTPase<br />
BEM1<br />
SH3 domains<br />
Diploid bud-site selection<br />
R<strong>and</strong>om budding in diploids/mo<strong>the</strong>r cells<br />
ACT1<br />
Actin<br />
SPA2<br />
Coiled-coil domain<br />
RSV161, RSV167<br />
SH3 domain<br />
BNI1, BUD6, BUD7 ?<br />
BUD8 ?<br />
BUD9 ?<br />
Septin-ring<br />
Bipolar budding in haploids<br />
CDC3, CDC<strong>10</strong>, CDC11, CDC12 <strong>10</strong>-nm filament ring components<br />
GTP-binding domain<br />
Budding is not a r<strong>and</strong>omized, uncontrolled process; cellular geometry is explicitly important in<br />
localizing budding sites. Numerous studies have endeavoured to explain at <strong>the</strong> cellular <strong>and</strong> molecular<br />
level how polarized cell growth is regulated <strong>and</strong> how <strong>the</strong> site of emerging buds is chosen. Bud site<br />
selection depends on several physiological <strong>and</strong> genetic factors. For example, cell mating type is<br />
important, <strong>and</strong> a <strong>and</strong> α haploid cells are kwon to exhibit an axial budding pattern, where as<br />
a/α diploids exhibit a bipolar budding pattern. Axial budding means that mo<strong>the</strong>r <strong>and</strong> daughter cells<br />
form a new bud near <strong>the</strong> preceding bud scar <strong>and</strong> birth scar, respectively. Bipolar budding is when<br />
daughter cells bud firstly away from <strong>the</strong>ir mo<strong>the</strong>r, while mo<strong>the</strong>r cells ei<strong>the</strong>r bud away or toward<br />
daughter cells.<br />
The hierarchy of cell polarity is governed by <strong>the</strong> interplay of various genes that dictate <strong>the</strong> orientation<br />
of cytoskeletal elements. For example, <strong>the</strong> bud-site selection genes (BUD genes) are required for<br />
determining <strong>the</strong> orientation of actin fibres, <strong>and</strong> genes for bud formation (such as CDC24, CDC42,<br />
BEM1) direct cell surface growth to <strong>the</strong> developing bud. Budding is strictly connected to events in <strong>the</strong><br />
cell cycle (see below) in that cyclins <strong>and</strong> cyclin-dependent kinases play a decisive role in actin<br />
assembly <strong>and</strong> in localizing <strong>and</strong> timing of bud emergence.<br />
It may be mentioned briefly that fission yeasts, like Schizosaccharomyces pombe, divide exclusively<br />
by forming a cell septum analogous to <strong>the</strong> mammalian cell cleavage furrow, which constricts <strong>the</strong> cell<br />
into two equal-sized daughters.<br />
<strong>10</strong>.1.2 <strong>Yeast</strong> Septins<br />
The septin proteins assemble into filaments that lie underneath <strong>the</strong> plasma membrane. In<br />
Saccharomyces cerevisiae, where <strong>the</strong>y were first identified, septins are visible as electron-dense<br />
cortical rings at <strong>the</strong> mo<strong>the</strong>r bud neck. In multicellular organisms, <strong>the</strong>y are found at <strong>the</strong> cleavage furrow<br />
<strong>and</strong> o<strong>the</strong>r cortical locations. Consistent with <strong>the</strong>ir localization, septins have been shown to be required<br />
for cytokinesis in yeast, Drosophila melanogaster, <strong>and</strong> mammalian cells.
Septins are highly conserved cytoskeletal elements found in fungi, mammals, <strong>and</strong> all eukaryotes<br />
examined thus far, with <strong>the</strong> exception of plants [Barral et al., 2000; Casamayor & Snyder, 2004].<br />
Recent evidence in yeast has demonstrated that septins participate in a variety of o<strong>the</strong>r cellular<br />
processes, including cell morphogenesis, bud site selection, chitin deposition, cell cycle regulation, cell<br />
compartmentalization, <strong>and</strong> spore wall formation. Since septins participate in many cellular processes,<br />
it is not surprising that a diverse set of proteins have been found associated with <strong>the</strong> yeast septin<br />
cytoskeleton.<br />
The septins have a highly conserved structure. They contain a central GTP-binding domain flanked by<br />
a basic region at <strong>the</strong> amino terminus, <strong>and</strong> most septins contain a coiled-coil domain at <strong>the</strong> carboxy<br />
terminus. In yeast, five septins, Cdc3, Cdc<strong>10</strong>, Cdc11, Cdc12, <strong>and</strong> Shs1, localize to <strong>the</strong> mo<strong>the</strong>r bud<br />
neck in vegetatively growing cells. Cdc3 <strong>and</strong> Cdc12 are essential for growth at all temperatures,<br />
whereas Cdc<strong>10</strong> <strong>and</strong> Cdc11 are required only at elevated temperatures. Shs1 is a nonessential septin.<br />
<strong>Cell</strong>s containing temperature- sensitive mutations in ei<strong>the</strong>r CDC3, CDC<strong>10</strong>, CDC11, or CDC12 delay at<br />
a G2 checkpoint <strong>and</strong> arrest at <strong>the</strong> restrictive temperature, forming extensive chains of highly elongated<br />
cells.<br />
Figure <strong>10</strong>-4: Functions of septins.<br />
Table <strong>10</strong>-2: Diversity of septin expression <strong>and</strong> function.<br />
Gene Function Localization Biochemistry<br />
CDC3<br />
CDC<strong>10</strong><br />
CDC11<br />
CDC12<br />
Bud neck, site of<br />
bud emergence,<br />
base of schmoo<br />
Essential for cytokinesis <strong>and</strong><br />
polar-bud growth control. Cdc3p<br />
<strong>and</strong> Cdc12p required for viability,<br />
but not Cdc<strong>10</strong>p <strong>and</strong> Cdc11pin<br />
some backgrounds. Reqired for<br />
proper regulation of <strong>the</strong> Gin4p <strong>and</strong><br />
Hsl1p kinases<br />
SEP7 Required in vivo for proper<br />
regulation of Gin4p kinase<br />
Bud neck, site of<br />
bud emergence<br />
SPR3 Sporulation efficiency Prospore wall No data<br />
SPR28 No obvious phentype Prospore wall No data<br />
Found in 370 kDa complex<br />
that can form filaments in<br />
vitro<br />
Can complex with Cdc3p,<br />
Cdc<strong>10</strong>, Cdc11p, Cdc12p
Table <strong>10</strong>-3: Budding yeast septin-protein interations.<br />
Protein Function Septindependent<br />
localization<br />
Gin4p<br />
Hsl1<br />
Kcc4<br />
Bni4p<br />
Chs3p<br />
Chs4p<br />
Yck1p<br />
Yck2p<br />
Bud3p<br />
Bud4p<br />
Spa2p<br />
Protein kinases that function in<br />
septin localization <strong>and</strong> cell cycle<br />
progression<br />
Reqired for normal chitin<br />
deposition<strong>and</strong> morphology<br />
Required for normal chitin<br />
syn<strong>the</strong>sis<br />
Casein kinase I homologs,<br />
required for septin localization,<br />
cytokinesis, morphogenesis <strong>and</strong><br />
endocytosis<br />
Bud site selection<br />
Yes<br />
Yes<br />
Yes<br />
Genetic/physical<br />
interactions<br />
Gin4p interacts<br />
physically <strong>and</strong><br />
genetically with septins<br />
Two-hybrid interaction<br />
with Cdc<strong>10</strong>p <strong>and</strong> Chs4p<br />
Syn<strong>the</strong>tic lethal<br />
interaction between<br />
Chs4p <strong>and</strong> Cdc12p<br />
Localization<br />
Bud neck, site of bud<br />
emergence<br />
Bud neck<br />
Bud neck, site of bud<br />
emergence<br />
No Unknown Bud neck, sites of<br />
polarized growth, plasma<br />
membrane<br />
Yes<br />
Yes<br />
??<br />
Unknown<br />
Unknown<br />
Syn<strong>the</strong>tic lethal<br />
interaction with Cdc<strong>10</strong>p<br />
Bud neck, site of bud<br />
emergence<br />
Bni1p Cytokinesis/morphogenesis ?? Syn<strong>the</strong>tic lethal<br />
Bud tip<br />
interaction with Cdc12p<br />
Myo1p Type II myosin. Plays a role in Yes Unknown Bud neck<br />
cytogenesis<br />
Arf1p Morphogenesis during mating ?? Two-hybrid interaction<br />
with Cdc12p<br />
Base of mating projections<br />
<strong>10</strong>.1.3 <strong>Yeast</strong> Spindle Pole Body Dynamics<br />
The spindle pole body (SPB) is <strong>the</strong> sole site of microtubule organization in <strong>the</strong> budding yeast<br />
Saccharomyces cerevisiae. SPBs are embedded in <strong>the</strong> nuclear envelope throughout <strong>the</strong> yeast life<br />
cycle <strong>and</strong> are <strong>the</strong>refore able to nucleate both nuclear <strong>and</strong> cytoplasmic microtubules. The small size of<br />
<strong>the</strong> yeast SPB, its location in a membrane, <strong>and</strong> <strong>the</strong> fact that nearly all genes involved in SPB function<br />
are essential have presented significant challenges in its analysis. Never<strong>the</strong>less, <strong>the</strong> SPB is perhaps<br />
<strong>the</strong> best-characterized microtubule organizing center (MTOC).<br />
Nucleation of microtubules by eukaryotic microtubule organizing centers is required for a variety of<br />
functions, including chromosome segregation during mitosis <strong>and</strong> meiosis, cytokinesis, fertilization,<br />
cellular morphogenesis, cell motility, <strong>and</strong> intracellular trafficking. Analysis of MTOCs from different<br />
organisms shows that <strong>the</strong> structure of <strong>the</strong>se organelles is widely varied even though <strong>the</strong>y all share <strong>the</strong><br />
function of microtubule nucleation. Despite <strong>the</strong>ir morphological diversity, many components <strong>and</strong><br />
regulators of MTOCs, as well as principles in <strong>the</strong>ir assembly, seem to be conserved [Segal et al.,<br />
2001; Jaspersen & Winey, 2004; Cheeseman & Desay, 2004].<br />
The SPB is a cylindrical organelle that appears to consist of three disks or plaques of darkly staining<br />
material (Figure <strong>10</strong>-5): an outer plaque that faces <strong>the</strong> cytoplasm <strong>and</strong> is associated with cytoplasmic<br />
microtubules, an inner plaque that faces <strong>the</strong> nucleoplasm <strong>and</strong> is associated with nuclear microtubules<br />
<strong>and</strong> a central plaque that spans <strong>the</strong> nuclear membrane. One side of <strong>the</strong> central plaque is associated<br />
with an electron-dense region of <strong>the</strong> nuclear envelope termed <strong>the</strong> half-bridge. This is <strong>the</strong> site of new<br />
SPB assembly because darkly staining material similar in structure to <strong>the</strong> SPB accumulates on its<br />
distal, cytoplasmic tip during G1 phase of <strong>the</strong> cell cycle.
Careful analysis of SPB size <strong>and</strong> structure indicates that <strong>the</strong> SPB is a dynamic organelle. In haploid<br />
cells, <strong>the</strong> SPB grows in diameter from 80 nm in G1 to 1<strong>10</strong> nm in mitosis. The molecular mass of a<br />
diploid SPB, including microtubules <strong>and</strong> microtubule associated proteins, is estimated to be 1–1.5<br />
GDa. However, only 17 components of <strong>the</strong> mitotic SPB have been identified to date (Table <strong>10</strong>-4).<br />
Table <strong>10</strong>-4: <strong>Yeast</strong> spindle pole body components.<br />
Protein SPB location Role in SPB function<br />
Tub4 γ-tubulin complex MT nucleation<br />
Spc98 γ-tubulin complex MT nucleation<br />
Spc97 γ-tubulin complex MT nucleation<br />
Spc72 OP, HB γ-tubulin binding protein<br />
Nud1 OP, satellite MEN signalling<br />
Cnm67 IL1, OP, satellite Spacer, anchors OP to CP<br />
Spc42 IL2, OP, satellite Structural SPB core<br />
Spc29 CP, satellite Structural SPB core<br />
Cmd1 CP Structural Spc1<strong>10</strong> binding protein<br />
Spc1<strong>10</strong> CP to IP Spacer, γ-tubulin binding protein<br />
Ndc1 SPB periphery Membrane protein, SPB insertion<br />
Mps2 SPB periphery Membrane protein, SPB insertion<br />
Bbp1 SPB periphery SPB core, HB linker to membrane<br />
Kar1 HB Membrane protein, SPB duplication<br />
Mps3 HB Membrane protein, SPB duplication<br />
Cdc31 HB SPB duplication<br />
Sfi1 HB SPB duplication<br />
Mpc54 MP Replace Spc72 in meosis I<br />
Spo21 MP Replace Spc72 in meosis II<br />
CP= Central plaque; HB=half-bridge; IL1=Inner layer 1; IL2= Inner layer 2; IP=Inner plaque;<br />
MEN= Microtubule envelope; cMT= Cytoplasmic MT (microtubule); nMT= Nuclear MT; OP=Outer plaque.<br />
Figure <strong>10</strong>-5: Location of protein components of <strong>the</strong> spindle pole body.
Regulators of SPB duplication <strong>and</strong> function associate with <strong>the</strong> SPB during all or part of <strong>the</strong> cell cycle.<br />
Mps1, a conserved protein kinase required for multiple steps in SPB duplication <strong>and</strong> also for <strong>the</strong><br />
spindle checkpoint, localizes to SPBs <strong>and</strong> to kinetochores<br />
Figure <strong>10</strong>-6: SPB duplication pathway.<br />
SPB duplication (Figure <strong>10</strong>-6) can be divided into three steps: (1) half-bridge elongation <strong>and</strong><br />
deposition of satellite material, (2) expansion of <strong>the</strong> satellite into a duplication plaque <strong>and</strong> retraction of<br />
<strong>the</strong> half-bridge, <strong>and</strong> (3) insertion of <strong>the</strong> duplication plaque into <strong>the</strong> nuclear envelope <strong>and</strong> assembly of<br />
<strong>the</strong> inner plaque. Following completion of SPB duplication, <strong>the</strong> bridge connecting <strong>the</strong> side-by-side<br />
SPBs is severed, <strong>and</strong> SPBs move to opposite sides of <strong>the</strong> nuclear envelope (4). The requirements for<br />
various gene products in each step are shown in <strong>the</strong> figure. SPC72, NUD1, <strong>and</strong> CNM67 are probably<br />
required for step 2. SPBs are not syn<strong>the</strong>sized de novo. Consequently, every time a cell divides it must<br />
duplicate its SPB, as well as its genome, to ensure that both <strong>the</strong> mo<strong>the</strong>r <strong>and</strong> daughter cell contain one<br />
copy of all 16 chromosomes <strong>and</strong> one SPB. SPB duplication occurs in G1 phase of <strong>the</strong> cell cycle;<br />
however, defects in SPB duplication are not detected until mitosis when cells fail to form a functional<br />
bipolar spindle. Generally, SPB defects cannot be reversed at this point, so cells will eventually<br />
attempt chromosome segregation with a monopolar spindle, which results in progeny with aberrant<br />
DNA content <strong>and</strong>/or SPB number. Therefore, accurate SPB duplication during G1 is essential to<br />
maintain genomic stability.<br />
<strong>10</strong>.2 The <strong>Yeast</strong> <strong>Cell</strong> <strong>Cycle</strong><br />
<strong>10</strong>.2.1 General<br />
The cell cycle can be defined as <strong>the</strong> period between division of a mo<strong>the</strong>r cell <strong>and</strong> subsequent division<br />
of its daughter progeny. The regulatory mechanisms that order <strong>and</strong> coordinate <strong>the</strong> progress of <strong>the</strong> cell<br />
cycle have been intensely studied [overviews: Mala & Nurse, 1998; Futcher, 2000; Lauren et al.,<br />
2001]. Numerous proteins that have been characterized through mutations are collectively designated<br />
as cell division cycle (Cdc) proteins.<br />
The eukaryotic cell cycle involves both continuous events (cell growth) <strong>and</strong> periodic events (DNA<br />
syn<strong>the</strong>sis <strong>and</strong> mitosis). Commencement <strong>and</strong> progression of <strong>the</strong>se events in yeast can formally been<br />
distinguished into pathways for DNA syn<strong>the</strong>sis <strong>and</strong> nuclear division, spindle formation, bud emergence<br />
<strong>and</strong> nuclear migration, <strong>and</strong> cytokinesis. However, from a molecular viewpoint <strong>the</strong>se processes are<br />
intimately coupled (Figure <strong>10</strong>-6).
Figure <strong>10</strong>-7: <strong>Cell</strong> cycle phases <strong>and</strong> physiological processes. (Modified from Hartwell, 2002).<br />
<strong>10</strong>.2.1.1 Some Historical Notes<br />
Early attention towards <strong>the</strong> ‘biology of <strong>the</strong> cell cycle’ arose from a book written by J.M. Mitchison which<br />
appeared in 1971 [Mitchison, 1971]. It was Lee Hartwell, who made <strong>the</strong> decisive step by introducing<br />
<strong>the</strong> budding yeast, S. cerevisiae, as an experimental system into this field <strong>and</strong> by characterizing a<br />
number of genes involved in cell division <strong>and</strong> cell cycle control (cf. Figure <strong>10</strong>-7), dubbed CDC genes<br />
[e.g., Hartwell et al., 1970; Hartwell, 1971; 1973; 1974]. He <strong>and</strong> his collaborators arrived at this issue<br />
after research on protein syn<strong>the</strong>sis <strong>and</strong> ribosome syn<strong>the</strong>sis in yeast [e.g., Hartwell, 1967; McLaughlin<br />
& Hartwell, 1969; Hartwell et al., 1970] as well as on studies of mating <strong>and</strong> mating pheromones in<br />
yeast (see below) carried out between 1967 <strong>and</strong> 1970. Research on <strong>the</strong> yeast cell cycle included work<br />
on synchronization of haploid yeast cells as a prelude to conjugation [Hartwell, 1973], on segregation<br />
[Wood & Hartwell, 1982] <strong>and</strong> <strong>the</strong> spindle checkpoint [Hartwell & Weinert, 1989; Paulovich et al., 1997].<br />
In 1980, Kim Nasmyth, whose fields comprised <strong>the</strong> mating phenomena in S. cerevisiae as well as <strong>the</strong><br />
cell cycle, succeeded in isolating cell cycle genes by molecular cloning [Nasmyth & Reed, 1980].<br />
Soon after Hartwell’s approach, <strong>the</strong> group of Paul Nurse chose to introduce <strong>the</strong> fission yeast, S.<br />
pombe, to this field as a fur<strong>the</strong>r simple model organism [Nurse et al., 1976] <strong>and</strong> to follow Hartwell’s<br />
approach by isolating Cdc mutants in fission yeast. The first mutants collected were mainly defective in<br />
<strong>the</strong> events of mitosis <strong>and</strong> cell division <strong>and</strong> subsequent screens carried out toge<strong>the</strong>r with Kim Nasmyth<br />
identified more mutants defective in S-phase [Nurse et al., 1976]. One important early finding was <strong>the</strong><br />
presence of a yeast homolog in S. pombe, cdc2, which later was shown to functionally correspond to<br />
<strong>the</strong> yeast CDC28 gene [Beach et al., 1982]. Fur<strong>the</strong>r, <strong>the</strong>re was <strong>the</strong> S. pombe wee1 gene that acted in<br />
G2 <strong>and</strong> controlled <strong>the</strong> cell cycle timing of mitosis [Nurse & Thuriaux, 1980]. Surprisingly, fur<strong>the</strong>r<br />
experiments disclosed that cdc2 was unusual in being required twice during <strong>the</strong> cell cycle, first in G1<br />
for onset of S-phase <strong>and</strong> <strong>the</strong>n again in G2 for onset of mitosis [Nurse & Bissett, 1981]. Obviously,<br />
S.pombe cdc2 had a central role controlling <strong>the</strong> fission yeast cell cycle. In G1 it was required to<br />
execute onset of S-phase, <strong>and</strong> in G2 it acted as a major rate limiting step determining <strong>the</strong> onset of<br />
mitosis. Next, <strong>the</strong> cdc2 gene product was identified as a kinase [Simanis & Nurse, 1986] <strong>and</strong> shown to
undergo tyrosine phosphorylation at <strong>the</strong> G2/M transition [Gould & Nurse, 1989]. A fur<strong>the</strong>r important<br />
player in control of <strong>the</strong> cell cycle turned out to be <strong>the</strong> Cdc13p cyclin, <strong>the</strong> level of which varied during<br />
<strong>the</strong> cell cycle, <strong>and</strong> which was required for Cdc2 protein kinase activation [Moreno et al., 1989].<br />
The proposition that cell cycle control was conserved in yeast <strong>and</strong> humans, <strong>and</strong> probably in all<br />
eukaryotes, was substantiated by isolating <strong>and</strong> characterizing equivalents for cdc2 [Lee & Nurse,<br />
1987]. The speculation was that human CDC2 might act at two points in <strong>the</strong> cell cycle, at <strong>the</strong> ‘G1<br />
restriction point’ known to operate in mammalian cells, <strong>and</strong> at <strong>the</strong> G2/M transition where it served as<br />
‘maturation promoting factor’ (MPF) known to control M-phase in metazoan eggs <strong>and</strong> oocytes [Nurse,<br />
1990]. These two functions accentuated <strong>the</strong> importance of cyclin-dependent kinases (CDKs) in<br />
regulating <strong>the</strong> orderly progression through S-phase <strong>and</strong> mitosis during <strong>the</strong> cell cycle. The onset of S-<br />
phase requires two sequential steps: <strong>the</strong> first one is only operative if CDK activity is absent (i.e. in<br />
early G1) whilst <strong>the</strong> second requires <strong>the</strong> presence of CDK activity, which later appears at <strong>the</strong> G1/S<br />
boundary, thus allowing progression through step two <strong>and</strong> bringing about <strong>the</strong> initiation of S-phase<br />
[Wuarin & Nurse, 1996]. During G2 <strong>the</strong> continued presence of CDK activity prevents step one from<br />
occurring again <strong>and</strong> this blocks onset of a fur<strong>the</strong>r S-phase. At <strong>the</strong> G2/M boundary, a fur<strong>the</strong>r increase in<br />
CDK activity triggers mitosis. Exit from mitosis <strong>and</strong> <strong>the</strong> ending of <strong>the</strong> cell cycle <strong>the</strong>refore requires<br />
destruction of CDK activity, <strong>and</strong> because <strong>the</strong> subsequent G1 cells lack CDK activity <strong>the</strong>y are able to<br />
carry out step one for S-phase <strong>and</strong> <strong>the</strong> whole series of events can be repeated [Stern & Nurse, 1996].<br />
On <strong>the</strong> long run, <strong>the</strong> findings from <strong>the</strong> two yeast models obviously had to be complemented by<br />
observations made in o<strong>the</strong>r organisms. An underst<strong>and</strong>ing what actually “drives” <strong>the</strong> cell cycle, came<br />
from most important discoveries in aquatic organisms: In 1980, Wu & Gerhart [1980] purified <strong>the</strong><br />
maturation promoting factor (MPF) from Xenopus eggs. Tim Hunt, who started research on <strong>the</strong> cell<br />
cycle <strong>the</strong> same year, was soon able to show <strong>the</strong> existence of <strong>the</strong> cyclins in sea urchins [Evans et al.,<br />
1982; Evans et al., 1983].<br />
Though many research groups contributed to this field, Hartwell, Hunt, <strong>and</strong> Nurse were honoured for<br />
<strong>the</strong>ir pioneering work <strong>and</strong> outst<strong>and</strong>ing discoveries in cell cycle research by awarding <strong>the</strong>m <strong>the</strong> Nobel<br />
Prize in 2001 [Hartwell, 2002; Nurse, 2001; Hunt, 2001]. The general principles come clear from Nurse<br />
[2000]: “There are several control points during <strong>the</strong> cell cycle: in late G1, called Start in yeast or <strong>the</strong><br />
Restriction point in mammals, in late G2 <strong>and</strong> just prior to anaphase. To pass each point, cells have to<br />
fulfil several prerequisites. Before passing Start, cells can undergo two developmental programmes,<br />
i.e. entry into <strong>the</strong> mitotic cell cycle or sexual development. Adequate nutritional conditions <strong>and</strong> a critical<br />
cell size are required to traverse Start. During G2, cells have to check whe<strong>the</strong>r DNA replication is<br />
completed <strong>and</strong> ensure that DNA is not damaged. Before chromosome separation cells also examine<br />
whe<strong>the</strong>r chromosomes are aligned <strong>and</strong> spindles are formed properly. These cell-cycle checkpoints are<br />
<strong>the</strong> mechanisms that govern <strong>the</strong> order of <strong>the</strong> cell-cycle events, because if <strong>the</strong> order of <strong>the</strong> events is<br />
incorrect <strong>the</strong>n a full complement of genetic information is not transmitted at cell division, which may<br />
lead to cancer in higher eukaryotes. Much is now known about regulation of <strong>the</strong> eukaryotic cell cycle<br />
but two areas have yet to be fully understood. The first area is concerned with <strong>the</strong> molecular<br />
mechanisms acting during <strong>the</strong> DNA-replication <strong>and</strong> DNA-damage checkpoints, which block mitosis if<br />
DNA replication is incomplete or DNA is damaged. The second concerns <strong>the</strong> controls which operate<br />
during <strong>the</strong> meiotic cell cycle.”
<strong>10</strong>.2.1.2 Periodic Events in <strong>the</strong> <strong>Cell</strong> <strong>Cycle</strong><br />
The periodic events can be divided into four phases (cf. Figure <strong>10</strong>-7): DNA syn<strong>the</strong>sis (S phase); a<br />
post-syn<strong>the</strong>tic gap (G2 phase); mitosis (M phase); <strong>and</strong> a pre-syn<strong>the</strong>tic gap (G1 phase). For division,<br />
yeast cells must reach a critical size. The key point in control of <strong>the</strong> cell cycle is START, <strong>the</strong> transition<br />
that initiates processes like DNA syn<strong>the</strong>sis in S phase, budding <strong>and</strong> spindle pole body duplication.<br />
Once cells have passed START, <strong>the</strong>y are irreversibly committed to replicating <strong>the</strong>ir DNA <strong>and</strong><br />
progressing through <strong>the</strong> cell cycle. START thus coordinates <strong>the</strong> cell cycle with cell growth. Nutrient<br />
starvation as well as induction of mating blocks passage through START. There are additional<br />
checkpoints that arrest cells during <strong>the</strong> cell cycle to avoid DNA damage or cell death due to events<br />
occurring out of order. These control points are situated at <strong>the</strong> G1-S <strong>and</strong> G2-M boundaries <strong>and</strong> can be<br />
considered as internal regulatory systems that arrest <strong>the</strong> cell cycle if prerequisites for progression are<br />
not met.<br />
After having passed <strong>the</strong> cell-size dependent START checkpoint, <strong>the</strong> level of cyclins (Cln,Clb)<br />
dramatically increase. Cyclins are periodically expressed <strong>and</strong> different cyclins (at least 11 in yeast)<br />
are known to be involved in <strong>the</strong> control of G1 (G1 cyclins), G2 (B-type cyclins) <strong>and</strong> DNA syn<strong>the</strong>sis (S<br />
phase cyclins). G1 cyclins are transcriptionally regulated. Cln3p, a particular G1 cyclin, is a putative<br />
sensor of cell size, which acts by modulating <strong>the</strong> levels of o<strong>the</strong>r cyclins. In addition to cyclin<br />
accumulation, <strong>the</strong> activity of a cylin-dependent kinase (CDK) which is an effector of START, is<br />
induced; this is <strong>the</strong> gene product of CDC28. Cdc28p (also termed Cdk1p) couples with G1 cyclins that<br />
activate its kinase potential. Homologues of this 34 kDa protein have been characterized in o<strong>the</strong>r<br />
eukaryotes. Cdk1 as well as being essential for S phase, is also important in controlling entry into<br />
mitosis. Complex formation of Cdk1 has been established with Cln1-3 (at G1), with Clb5,6 (at S), Clb<br />
3,4 (at S/G2) <strong>and</strong> Clb1,2 (at M). Alternation of cell cycle phases appears to be due to mechanisms that<br />
one cyclin family succeeds ano<strong>the</strong>r. The level of cyclins are controlled by syn<strong>the</strong>sis <strong>and</strong> programmed<br />
proteolysis by <strong>the</strong> proteasome. In this regard it has been shown that G2 cyclins are necessary for<br />
degradation of G1 cyclins, <strong>and</strong> that G2 cyclin syn<strong>the</strong>sis is coupled to removal of G1 cyclins (Figure <strong>10</strong>-<br />
8).<br />
Important players in this game are inhibitor proteins, known as CKIs, which block CDK activity in G1.<br />
They represent a key mechanism by which <strong>the</strong> onset of DNA replication is regulated. One such<br />
inhibitor is Sic1p: upon its destruction by programmed proteolysis, cyclin-Cdk1 activity is induced. This<br />
degradation <strong>the</strong>n triggers <strong>the</strong> G1-S transition [Lauren et al., 2001].
Figure <strong>10</strong>-8: Regulation of <strong>the</strong> yeast cell cycle.<br />
In addition to this pathway, cell cycle progression is controlled by <strong>the</strong> availability of nutrients (Figure<br />
<strong>10</strong>-9). Nutrient levels (for example, glucose or nitrogenous compounds) regulate <strong>the</strong> intracellular<br />
concentration of cAMP via a small G protein, Ras. The so-called Ras/cAMP pathway is well<br />
documented (see chapter 13). Decreasing levels lead to G1 arrest, while increasing levels induce <strong>the</strong><br />
cAMP-dependent protein kinase (PKA), which <strong>the</strong>n phosphorylates <strong>and</strong> <strong>the</strong>reby activates specific<br />
transcription factors involved in START.<br />
Figure <strong>10</strong>-9: G1 regulation in yeast.
G2-M control is characterized by <strong>the</strong> association of Cdk1 with B-type cyclins. Complexing of <strong>the</strong> kinase<br />
with <strong>the</strong> cyclins activates <strong>the</strong> kinase, leading to induction of M phase. At <strong>the</strong> end of M phase, <strong>the</strong><br />
mitotic cyclins are removed by programmed proteolysis.<br />
<strong>10</strong>.2.2 DNA Replication<br />
Chromosome duplication is central to cell division <strong>and</strong> is tightly controlled during <strong>the</strong> cell cycle. In<br />
eukaryotic cells, chromosome duplication is accomplished by initiating replication forks at many origins<br />
of replication on each chromosome; activation of <strong>the</strong> different replication origins is coordinated<br />
during S phase [Donaldson et al., 1999; Stillman, 2001; Raghuraman et al, 2001; Iyer et al., 2001;<br />
Heun et al., 2001; Wyrick et al., 2001]. ARS elements as substantial elements in yeast replication<br />
origins have been discussed above (chapter 2).<br />
Figure <strong>10</strong>-<strong>10</strong>: Licensing by <strong>the</strong> origin recognition complex.<br />
A two-step model of replication initiation, in which origins are licensed for firing during G1 but only<br />
activated under cellular conditions that preclude <strong>the</strong>ir licensing, has been proposed. In yeast, <strong>the</strong> sixprotein<br />
origin recognition complex (ORC) remains bound to DNA throughout <strong>the</strong> cell cycle <strong>and</strong><br />
forms <strong>the</strong> core of <strong>the</strong> origin complex to which o<strong>the</strong>r protein components are recruited. Interestingly,<br />
ORC is also involved in silencing of gene expression. The first step in pre-replicative complex<br />
formation (Figure <strong>10</strong>-<strong>10</strong>) appears to be <strong>the</strong> recruitment of Cdc6p by ORC, after which Cdc6p is in turn<br />
required for loading of Mcm/P1 protein complexes onto DNA, resulting in <strong>the</strong> origin being licensed for<br />
replication in <strong>the</strong> subsequent S phase. Cdc6p is an ATPase, <strong>and</strong> ATP hydrolysis seems essential for<br />
<strong>the</strong> loading of Mcm/P1. The role of <strong>the</strong> Mcm/P1 gene products is not sufficiently clarified yet. This<br />
protein family consists of six members, all of which are involved in <strong>the</strong> licensing process but <strong>the</strong>ir<br />
biochemical function is unclear. It may well be that <strong>the</strong>y supply helicase function required in both<br />
initiation <strong>and</strong> elongation phases of replication. Following Cdk activation in late G1, Cdc45p becomes<br />
associated with <strong>the</strong> origin; removal of Cdc6p may stimulate this event.
Figure <strong>10</strong>-11: Regulation of replication by cyclins.<br />
Interestingly, DNA replication must be restricted to only one round in each cell cycle. Work in yeast<br />
has shown that Cdk activity during G2 is required to prevent re-replication of DNA; <strong>the</strong> mechanism of<br />
this inhibition is not known, only <strong>the</strong> fact that <strong>the</strong> Cdks are involved in promoting <strong>the</strong> degradation of<br />
Cdc6p.<br />
Progression through <strong>the</strong> cell cycle is highly coordinated. Replication origin firing during S phase is not<br />
r<strong>and</strong>om but ra<strong>the</strong>r is under strict temporal <strong>and</strong> spatial control. Replication forks cluster in discrete<br />
'replication factories' within <strong>the</strong> nucleus <strong>and</strong> components required for elongation associate with nuclear<br />
structural components such as <strong>the</strong> lamina. Definitely, early <strong>and</strong> late origins have to be distinguished.<br />
Factors that share responsibility for promoting S phase are two B type cyclins, Clb5p <strong>and</strong> Clb6p, in<br />
conjunction with a single cyclin-dependent kinase, Cdc28p. As it apperas, Clb5p is executing <strong>the</strong> origin<br />
firing programme in both early <strong>and</strong> late origins, while Clb6-Cdc28 can only fire early replication origins.<br />
Fur<strong>the</strong>r, <strong>the</strong> origin-firing programme is subject to checkpoint controls (Figure <strong>10</strong>-11). One of <strong>the</strong><br />
essential players is Rad53p, which is involved in monitoring successful execution of <strong>the</strong> programme of<br />
DNA replication during S phase, <strong>and</strong> co-ordinating a controlled arrest if problems are encountered.<br />
Rad53p also seems to be required for maintaining <strong>the</strong> level of nucleotides in <strong>the</strong> normal S phase.<br />
The six subunits of <strong>the</strong> ORC complex, which was shown to be also involved in transcriptional<br />
silencing, were isolated <strong>and</strong> characterized [Diffley & Cocker, 1992; Micklem et al., 1993; Foss et al.,<br />
1993; Rao & Stillman, 1995; Loo et al., 1995; Bell et al.,1995; Li et al., 1998; Du & Stillman, 2002].<br />
Prereplicative (or preinitiation) complexes are assembled during M <strong>and</strong> G1 phase by binding of <strong>the</strong>
ORC complex to <strong>the</strong> multiple ARS sequences, whereby this binding persists throughout <strong>the</strong> cell cycle.<br />
In late M phase, most importantly, <strong>the</strong> ATP-dependent protein Cdc6 is recruited by <strong>the</strong> ORC, which in<br />
turn promotes loading of <strong>the</strong> minichromosome-maintenance (MCM) complex on to chromatin [Liang et<br />
al., 1995; Williams et al., 1997; Zou et al., 1997; Weinreich et al., 1999; Weinreich et al., 2001;<br />
Raghuraman et al., 2001; Stillman, 2001; Stillman, 2005]. Activation of two protein kinase complexes,<br />
Cdc28-B cyclins <strong>and</strong> Cdc7-Dbf4, <strong>the</strong>n serves as <strong>the</strong> final signal activating replication fork movement,<br />
whereupon <strong>the</strong> DNA-replication machinery, including DNA polymerases <strong>and</strong> proliferating-cell nuclear<br />
antigen (PCNA), initiates DNA syn<strong>the</strong>sis (cf. Figure 2-13).<br />
<strong>10</strong>.2.3 Spindle Dynamics<br />
In S. cerevisiae, <strong>the</strong> mitotic spindle must orient along <strong>the</strong> cell polarity axis, defined by <strong>the</strong> site of bud<br />
emergence, to ensure correct nuclear division between <strong>the</strong> mo<strong>the</strong>r <strong>and</strong> daughter cells [Segal <strong>and</strong><br />
Bloom, 2001]. Establishment of spindle polarity dictates this process <strong>and</strong> relies on <strong>the</strong> concerted<br />
control of spindle pole function <strong>and</strong> a precise programme of cues originating from <strong>the</strong> cell cortex that<br />
directs <strong>the</strong> cytoplasmic microtubule attachments during spindle morphogenesis. This cues cross talk<br />
with <strong>the</strong> machinery responsible for bud site selection, indicating that orientation of <strong>the</strong> spindle is<br />
mechanistically coupled to <strong>the</strong> definition of a polarity axis <strong>and</strong> <strong>the</strong> division plane.<br />
Spindle morphogenesis in yeast is initiated by <strong>the</strong> execution of START at <strong>the</strong> G1-S transition of <strong>the</strong><br />
cell cycle. Progression through START triggers bud emergence, DNA replication <strong>and</strong> <strong>the</strong> duplication of<br />
<strong>the</strong> microtubule-organizing centre (MTOC) - <strong>the</strong> spindle pole body (SPB) (Figures <strong>10</strong>-5 <strong>and</strong> <strong>10</strong>-12).<br />
The single stages of mitosis <strong>and</strong> intracellular movements can be distinguished by time-lapse phasecontrast<br />
microscopy. In addition to <strong>the</strong> polymerization <strong>and</strong> depolymerization of tubulin (<strong>the</strong> major<br />
microtubular protein), cytolasmic dynein is a mechanochemical enzyme or motor protein which drives<br />
microtubules motility in yeast. Actin filaments, ei<strong>the</strong>r as cytoskeletal cables or as cortical membrane<br />
patches, undergo dynamic changes during <strong>the</strong> cell cycle. The microtubules emanate from <strong>the</strong> SPBs<br />
toward <strong>the</strong> new bud <strong>and</strong> orientate <strong>the</strong> nucleus <strong>and</strong> intranuclear spindle at mitosis. The nuclear<br />
membrane remains intact throughout mitosis with <strong>the</strong> mitotic spindle forming intranuclearly between<br />
two SPBs embedded in <strong>the</strong> nuclear envelope. Once <strong>the</strong> genome replicates, <strong>the</strong> spindle aligns parallel<br />
to <strong>the</strong> mo<strong>the</strong>r bud axis <strong>and</strong> elongates eventually to provide each cell with one nucleus.<br />
The program for <strong>the</strong> establishment of spindle polarity, primed by cellular factors partioning<br />
asymmetrically between <strong>the</strong> bud <strong>and</strong> <strong>the</strong> mo<strong>the</strong>r cortex, coupling of this process to bud site selection<br />
<strong>and</strong> polarized growth has been elucidated in some detail [Segal & Bloom, 2001]. Several cortical<br />
components implicated in spindle orientation such as Bni1p, a target of <strong>the</strong> polarizing machinery<br />
essential in bud site selection <strong>and</strong> spindle orientation, <strong>and</strong> <strong>the</strong> actin interactor Aip3p/Bud6p are initially<br />
localized to <strong>the</strong> bud tip. O<strong>the</strong>r cortical elements (e.g. Num1p) are restricted initially to <strong>the</strong> mo<strong>the</strong>r cell<br />
during spindle assembly.
Figure <strong>10</strong>-12: Spindle dynamics.<br />
Figure <strong>10</strong>-13: Fluorescence imaging of microtubules.<br />
Factors mediating <strong>the</strong> process of microtubule attachment with <strong>the</strong> bud cell cortex are Bim1p <strong>and</strong><br />
Kar9p. Bim1p can directly bind to microtubules <strong>and</strong> is required for <strong>the</strong> high dynamic instability of<br />
microtubules that is characteristic of cells before spindle assembly. Kar9p has been implicated in <strong>the</strong><br />
orientation of functional microtubule attachments into <strong>the</strong> bud during vegetative growth. It is delivered<br />
to <strong>the</strong> bud by a Myo2-dependent mechanism presumably tracking on actin cables. Interaction of <strong>the</strong><br />
two factors, Bim1p <strong>and</strong> Kar9p, appears to provide a functional linkage between <strong>the</strong> actin <strong>and</strong><br />
microtubule cytoskeletons. In addition, Bud3p, a protein for axial budding of haploid cells, accumulates<br />
at <strong>the</strong> bud neck <strong>and</strong> is required for <strong>the</strong> efficient association of Bud6p to <strong>the</strong> neck region. Fur<strong>the</strong>r, a<br />
variety of motor proteins are necessary in spindle morphogenesis: dynein <strong>and</strong> <strong>the</strong> kinesin-like proteins<br />
Kip2p <strong>and</strong> Kip3p, as well as Kar3p are involved in regulating microtubule dynamics, mediating nuclear<br />
migration to <strong>the</strong> bud neck <strong>and</strong> facilitating spindle translocation (Figure <strong>10</strong>-13).<br />
<strong>10</strong>.2.4 Telomere Replication<br />
Several factors have been found to be implicated in stabilising telomeres <strong>and</strong> regulating telomere<br />
length; one of <strong>the</strong> earliest identified was Rap1, binding to both silencer <strong>and</strong> activator elements [Shore<br />
& Nasmyth, 1987; Shore et al., 1987; Kurtz & Shore, 1991]. Many discoveries in this field go back to<br />
<strong>the</strong> work of D. Shore <strong>and</strong> colleagues, who also found two proteins, Rif1 <strong>and</strong> Rif2, [Hardy et al., 1992;<br />
Wotton & Shore, 1997] <strong>and</strong> a SIR complex (Sir2,3 <strong>and</strong> 4) [Moretti et al., 1994] interacting with Rap1.
Remarkably <strong>the</strong>se factors are involved in telomere length regulation [Lustig et al., 1990; Hardy et al.,<br />
1992; Wotton & Shore, 1997; Shore, 2001, 2004]. Telomere length regulation is an issue for long<br />
discussed as a decisive phenomenon in cellular senescence <strong>and</strong> ageing [Shore, 1997, 1998; Smeal &<br />
Guarente, 1997; Blackburn et al., 2006] pertinent to all eukaryotic organisms.<br />
Because of <strong>the</strong>ir 'open-end' structure, telomeres have to be replicated by a specialized telomerase<br />
system [Cohn & Blackburn, 1995]. <strong>Yeast</strong> telomerase consists of <strong>the</strong> gene products of three EST genes<br />
(EST1, EST2, EST3) [Taggart et al., 2002; Taggart & Zakian, 2003; Lundblad, 2003], whereby Est2p<br />
acts as <strong>the</strong> catalaytic subunit, <strong>and</strong> an RNA component (TLC1) which is employed as a matrix in <strong>the</strong><br />
syn<strong>the</strong>sis of telomeric DNA [Brigati et al., 1993]. Additionally, telomere replication is dependent on:<br />
(i) <strong>the</strong> TRF1 complex, consisting of Ku70 (Hdf1) <strong>and</strong> Ku80 (Hdf2) proteins <strong>and</strong> interacting with<br />
Cdc13p; which is also crucial for non-homologous DNA-double str<strong>and</strong> repair <strong>and</strong> protects telomeres<br />
against nucleases <strong>and</strong> recombinases [e.g., Stellwagen et al., 2003; Fisher et al., 2004],<br />
(ii) a number of RAD proteins (Rad50, Rad51, Rad52),<br />
(iii) Sgs1p, a helicase, preventing deletirious recombination between telomeric sequences,<br />
(iv) <strong>and</strong> a number of o<strong>the</strong>r proteins such as Pif1p detected in Zakian’s group [Zhou et al., 2000].<br />
The participation of helicase Pif1p in telomere replication [Boule & Zakian, 2006] as well as <strong>the</strong><br />
involvement of <strong>the</strong> telomere replication apparatus in healing DNA breaks [Bianchi et al., 2004] have<br />
been solved in <strong>the</strong> budding yeast model (Figure <strong>10</strong>-14). Pif1p also helps flap elongation during<br />
Okazaki fragment maturation. Rrm3p, a helicase belonging to <strong>the</strong> Pif family, appears to be involved in<br />
replication fork progression [Boule & Zakian, 2006].<br />
Figure <strong>10</strong>-14: Models explaining Pif1p action at telomeres <strong>and</strong> during Okazaki<br />
fragment processing, <strong>and</strong> Rrm3p action during replication fork progression.<br />
(Reproduced from Boule <strong>and</strong> Zakian, 2006).<br />
In telomerase-deficient cells, telomeres shorten progressively (in about 60 generations) <strong>and</strong> lead to a<br />
shortening of telomeres <strong>and</strong> increased senescence. Two types of survival pathways are known to be<br />
induced upon defects in <strong>the</strong> telomerase system, which consist of telomere elongation by break-
induced replication (BIR): type I survivors maintain short TG1-3 repeats but amplify <strong>the</strong> Y' repeats, while<br />
type II survivors amplify <strong>the</strong> TG1-3 repeats to several kb in length but do not amplify <strong>the</strong> Y' elements.<br />
During chromosome replication in yeast, telomeres connect to <strong>the</strong> spindle pole bodies (SPBs), <strong>and</strong><br />
<strong>the</strong>re are multiple pathways for telomere te<strong>the</strong>ring [Taddei & Gasser, 2004].<br />
Recent reviews dealing with <strong>the</strong> mechanism of telomere replication <strong>and</strong> <strong>the</strong> participation of chromatin<br />
remodelling factors can be found in [Teixeira & Gilson, 2005; Gilson & Geli, 2007].<br />
<strong>10</strong>.2.5 Sister Chromatid Cohesion <strong>and</strong> Separation<br />
Sister chromatid cohesion is essential for accurate chromosome segregation during <strong>the</strong> cell cycle<br />
[Nasmyth, 1999; Biggins & Murray, 1999; Robert et al., ; Nasmyth, 2002; Camobel & Cohen-Fix;<br />
2002; Uhlmann, 2004]. A number of structural proteins are required for sister chromatid cohesion <strong>and</strong><br />
<strong>the</strong>re seems be a link in some organisms between <strong>the</strong> processes of cohesion <strong>and</strong> condensation.<br />
Likewise, a number of proteins that induce <strong>and</strong> regulate <strong>the</strong> separation of sister chromatids have been<br />
identified.<br />
As long ago as 1879, Fleming noticed that “<strong>the</strong> impetus causing nuclear threads to split longitudinally<br />
acts simultaneously on all of <strong>the</strong>m” [Fleming, 1879]. Chromosome splitting is an irreversible process<br />
<strong>and</strong> must <strong>the</strong>refore be highly regulated. Damage to <strong>the</strong> genome cannot easily be repaired by<br />
recombination nor can aberrant chromosome alignments be corrected, once sister chromatids<br />
separate from one ano<strong>the</strong>r. Therefore, sister chromatids have to be kept toge<strong>the</strong>r during mitosis after<br />
chromosome replication <strong>and</strong> <strong>the</strong>y have to be disentangled in metaphase before cell division starts in<br />
anaphase (Figure <strong>10</strong>-15). Keeping toge<strong>the</strong>r sister chromatids is absolutely required until all control<br />
mechanisms have been exerted that guarantee intactness of all replicated chromosomes.<br />
However, chromosomes do not remain inactive at this process: cohesion between sister chromatids<br />
generates <strong>the</strong> tension by which cells align <strong>the</strong>m on <strong>the</strong> metaphase plate. Cohesion also prevents<br />
chromosomes falling apart because of double-str<strong>and</strong>ed breaks <strong>and</strong> facilitates <strong>the</strong>ir repair by<br />
recombination.<br />
To date, we have ample knowledge of <strong>the</strong> molecular subtleties of how sister chromatids are kept<br />
toge<strong>the</strong>r <strong>and</strong> separated at appropriate moments of cell division, largely stemming from yeast as a<br />
model system. Before <strong>the</strong>se details were revealed, Koshl<strong>and</strong> & Hartwell [1987] had used SV40<br />
minichromosomes to show that <strong>the</strong> sister molecules are properly segregated when a cell cycle block is<br />
removed, demonstrating that sister minichromosome molecules need not remain topologically<br />
interlocked until anaphase in order to be properly segregated, <strong>and</strong> topological interlocking of sister<br />
DNA molecules apparently is not <strong>the</strong> primary force holding sister chromatids toge<strong>the</strong>r. This system has<br />
been kept since to study how sister chromatids behave during segragation [Ivanov & Nasmyth, 2005].<br />
Cohesion is mediated by a multisubunit complex called cohesin, which binds to chromosomes at<br />
multiple sites along chromosomes, from telophase until <strong>the</strong> onset of anaphase in <strong>the</strong> next cell cycle<br />
[Hirano, 2000; Nasmyth, 2001]. Cohesin consists of four core subunits, namely Smc1, Smc3, Scc1,<br />
<strong>and</strong> Scc3. Smc1 <strong>and</strong> Smc3 proteins (SMC complex) are characterized by 50-nm-long anti-parallel<br />
coiled coils flanked by a globular hinge domain <strong>and</strong> an ABC-like ATPase head domain. Whereas<br />
Smc1 <strong>and</strong> Smc3 heterodimerize via <strong>the</strong>ir hinge domains, <strong>the</strong> kleisin subunit Scc1 connects <strong>the</strong>ir<br />
ATPase heads, <strong>and</strong> this results in <strong>the</strong> formation of a large ring (Figure <strong>10</strong>-15). Cleavage of Scc1 by a<br />
cystein protease called ‘separin’ or ‘separase’ (Esp1 in yeast) triggers poleward movement of sisters
at <strong>the</strong> metaphase-to-anaphase transition (see below). Scc1 in turn recruits <strong>and</strong> binds <strong>the</strong> fourth<br />
cohesion subunit, Scc3, which has two orthologues in mammals.<br />
Figure <strong>10</strong>-16: Cohesins: <strong>the</strong> ‘glue’ between sister chromatids<br />
An early postulate was that cohesin connects sister DNA molecules through <strong>the</strong> binding of its two<br />
heads to each sister DNA molecule, thus forming a ‘glue’ between <strong>the</strong> sister chromatids [Toth et al.,<br />
1999; Anderson et al., 2002]. However, <strong>the</strong> finding that <strong>the</strong> N- <strong>and</strong> C-termini of Scc1 bind, respectively,<br />
to <strong>the</strong> Smc3 <strong>and</strong> Smc1 heads of <strong>the</strong> Smc1/Smc3 heterodimer [Haering et al., 2002] suggested that<br />
cohesin forms a large proteinaceous ring in which DNA str<strong>and</strong>s could be trapped (Figure <strong>10</strong>-15). It<br />
was concluded that <strong>the</strong> connection between sisters may be a topological ra<strong>the</strong>r than a chemical one<br />
[Nasmyth, 2002]. This hypo<strong>the</strong>sis turned out to be correct [e.g. Haering et al., 2002; Gruber et al.,<br />
2006]; it was confirmed <strong>and</strong> refined by experiments investigating <strong>the</strong> topological interaction between<br />
cohesin rings <strong>and</strong> a circular minichromosome [Ivanov & Nasmyth, 2005].<br />
With a diameter of close to 50 nm, <strong>the</strong> ring is sufficiently large to hold two sister DNA str<strong>and</strong>s toge<strong>the</strong>r<br />
even when wrapped around histones. At anaphase onset, it is <strong>the</strong> Scc1 subunit that is cleaved by<br />
separase, which in turn disrupts <strong>the</strong> interaction between <strong>the</strong> Smc heads in cohesin, thus opening <strong>the</strong><br />
ring [Weitzer et al., 2003; Uhlmann, 2003]. Critical to <strong>the</strong> cleavage of Scc1 is that its C-terminal<br />
cleavage product is quickly destroyed by targeted proteolysis [Rao et al., 2001] in order of preventing<br />
<strong>the</strong> Smc heads from interacting. Recently, it has been demonstrated that kleisin not only connects he<br />
Smc1 <strong>and</strong> Smc3 ATPase heads but also regulates <strong>the</strong>ir ATPase activity [Arumugam et al., 2006].<br />
For a long time, an unsolved problem was how <strong>the</strong> two DNA str<strong>and</strong>s are ‘entering’ <strong>the</strong> cohesin ring<br />
during (or after) DNA replication [Uhlmann, 2003]. Could <strong>the</strong> DNA replication fork simply slide through<br />
<strong>the</strong> cohesin rings that were put around DNA before S-phase? This would leave two replication<br />
products trapped inside <strong>the</strong> same ring without fur<strong>the</strong>r transport, <strong>and</strong> at <strong>the</strong> same time provide an<br />
intrinsic solution to <strong>the</strong> crucial requirement to only establish sister chromatid cohesion between<br />
au<strong>the</strong>ntic replication products <strong>and</strong> never between any o<strong>the</strong>r two sequences of DNA. It is in fact known<br />
that a number of yeast proteins, for example Eco1 (Cft7) [Skibbens et al., 1999; Toth et al., 1999;<br />
2000], or alternative chromatin remodeling complexes (RFC) [Mayer et al., 2001] as well as RSC<br />
[Huang et al., 2004] are required for efficient cohesion establishment during S-phase. These might<br />
even be regulators of a more sophisticated machinery which puts cohesin around both sister<br />
chromatids.
Recently this problem has come close to solution: Gruber et al. [2006] showed that cohesin’s hinges<br />
are not merely dimerization domains that are holding toge<strong>the</strong>r <strong>the</strong> cohesin ring by preventing kleisin’s<br />
dissociation from <strong>the</strong> SCM heads. Ra<strong>the</strong>r, entry of DNA into <strong>the</strong> cohesin ring requires opening <strong>and</strong><br />
transient dissociation of <strong>the</strong> Smc1 <strong>and</strong> Smc3 hinge domains [see also: Shintomi & Hirano, 2007].<br />
The binding of condensin (<strong>the</strong> second ‘glue’ complex) to chromatin has similarities with chromatin<br />
binding to cohesin [Lavoie et al., 2002]. Condensin, a 13 S complex, consists of two Smc proteins,<br />
Smc2p <strong>and</strong> Smc4p, <strong>and</strong> contains three o<strong>the</strong>r essential subunits, one of which is homologous to Scc1p;<br />
<strong>the</strong> newly found Smc5p-Smc6p complex preserves nuclear integrity [Torres-Rosell et al., 2005]. Just<br />
like cohesin, <strong>the</strong> topological structure of condensin is a ring. Condensin associates with chromatin<br />
independently of ATP, but ATP hydrolysis is needed for <strong>the</strong> binding reaction. A particular feat of<br />
condensin is that chromatin wraps around it, generating a torsion in <strong>the</strong> DNA [Wang et al., 2005]. Thus<br />
condensin is amenable of contributing to chromosome compaction; it also participates in DNA repair<br />
[Chen et al., 2004]. After partial removal of cohesin rings by <strong>the</strong> separase reaction, condensin replaces<br />
cohesin, both in mitosis <strong>and</strong> meiosis [Yu & Koshl<strong>and</strong>, 2005].<br />
What ensures ordered segregation <strong>and</strong> movement of chromatids to opposite poles of <strong>the</strong> cell?<br />
Segregation of chromosomes must be a tightly regulated process (cf. Figure <strong>10</strong>-16). The fraction of<br />
cohesin that persists on chromosomes until metaphase is responsible for holding sisters toge<strong>the</strong>r<br />
while <strong>the</strong>y bi-orient during prometaphase. It is an absolute requirement that <strong>the</strong> sister chromatids<br />
adopt an amphitelic orientation, i.e. that <strong>the</strong> spindle apparatus can be activated in a way that allows<br />
<strong>the</strong> traction of sister chromatids to opposite poles of <strong>the</strong> cell by <strong>the</strong> microtubules to occur. (Remember<br />
that in yeast microtubules connect kinetochores to spindle poles throughout <strong>the</strong> cell cycle).<br />
Sister chromatids are pulled to opposite 'halves' of <strong>the</strong> cell by microtubules that emanate from<br />
opposite spindle poles. These microtubules interdigitate <strong>and</strong> keep <strong>the</strong> two poles apart. Subsequently,<br />
a second set of microtubules attaches to chromosomes through specialized 'kinetochores' <strong>and</strong> pulls<br />
<strong>the</strong>m to <strong>the</strong> poles. In this way, sister chromatides separate <strong>and</strong> start to move into opposing poles.<br />
Figure <strong>10</strong>-16: Sister chromatid separation.
Two phases can be distinguished: (i) cohesin’s dissociation from <strong>and</strong> condensin’s association with<br />
chromosomes occurs in <strong>the</strong> complete absence of microtubules yet is capable of separating sister<br />
chromatids to ~0.5 µm. This first step in <strong>the</strong> individualisation process is triggered by <strong>the</strong> participation of<br />
PLK (‘Polo-like kinase’) which will phosphorylate cohesin (<strong>and</strong> possibly some o<strong>the</strong>r proteins). The<br />
second step, orienting sister chromatids on <strong>the</strong> mitotic spindle (Bi-orientation) <strong>and</strong> attaching<br />
microtubules to sister kinetochores, whereby chromosomes come under tension, is promoted by <strong>the</strong><br />
Aurora-like kinase Ipl1p <strong>and</strong> controlled by <strong>the</strong> spindle checkpoint (see below). (ii) The first clue to <strong>the</strong><br />
molecular basis for sister-chromatid separation in <strong>the</strong> second phase arose from <strong>the</strong> discovery of<br />
mitotic cyclins as proteins whose abundance fluctuates during embryonic-cleavage divisions [Evans et<br />
al., 1983] <strong>and</strong> that mitotic cyclins are regulatory subunits of a cyclin-dependent kinase (CDK1), whose<br />
activation in late G2 phase triggers mitosis. At commencement of anaphase, CDK activity is<br />
destructed by degrading of <strong>the</strong> cyclins [Glotzer et al., 1991], but is not required for <strong>the</strong> separation of<br />
sister chromatids [Surana et al., 1993; Dirick et al., 1995]. This suggested that proteolysis of fur<strong>the</strong>r<br />
proteins is necessary for sister chromatid separation [Holloway et al., 1993]. The apparatus<br />
responsible for targeting cyclin degradation turned out to be a highly conserved multi-subunit complex<br />
that possesses ubiquitin-protein ligase activity [Zachariae et al., 1998; Yu et al., 1998]. Initially named<br />
<strong>the</strong> cyclosome [Sudakin et al., 1995], <strong>the</strong> ligase moiety is now called <strong>the</strong> anaphase promoting<br />
complex (APC). It mediates <strong>the</strong> destruction of many proteins o<strong>the</strong>r than cyclins <strong>and</strong> was shown to be<br />
essential for <strong>the</strong> separation of sister chromatids [Irniger et al., 1995; King et al., 1995; Zachariae et. al.,<br />
1996; Michaelis et al., 1997; Guacci et al., 1997; Losada et al., 1998; Zachariae & Nasmyth, 1999].<br />
Ubiquitination mediated by APC/C requires rate-limiting activator proteins that bind to APC; in yeast<br />
<strong>the</strong>se are at least two proteins, namely Cdc20p <strong>and</strong> Cdh1p.These activators specify both substrate<br />
specificity <strong>and</strong> <strong>the</strong> timing of proteolysis. In <strong>the</strong> absence of APC/C function, yeast cells arrest in<br />
metaphase, <strong>and</strong> sister chromatids fail to segregate owing to <strong>the</strong> persistence of securin, <strong>the</strong> inhibitor of<br />
separase. Separase is activated by proteolysis of securin by APC/C.<br />
Once chromosomes have successfully bi-oriented, cells utilize an evolutionarily conserved machinery<br />
to initiate anaphase. Rapid disjunction of sister chromatids at anaphase requires cleavage of cohesin<br />
subunit Scc1 by a cysteine protease called separase (Esp1 in budding yeast), inactive through most of<br />
<strong>the</strong> cell cycle by its association with an inhibitor, securin [Yamamoto et al., 1996a; Ciosk et al., 1998;<br />
Uhlmann et al., 1999]. The metaphase-to-anaphase transition is triggered when securin (Pds1 in<br />
budding yeast [Cohen-Fix et al., 1996; Yamamoto et al., 1996b; Cohen-Fix & Koshl<strong>and</strong>, 1997]) is<br />
degraded by <strong>the</strong> proteasome following ubiquitination by <strong>the</strong> multicomponent E3 ubiquitin ligase known<br />
as <strong>the</strong> APC/C (cf. Figure 16). Securin accumulates within nuclei during late G1 phase, is maintained<br />
during G2 <strong>and</strong> early M phase, but degraded shortly before anaphase, so that separase becomes<br />
active. Separase resides in <strong>the</strong> cytoplasm until cells enter mitosis, whereupon it accumulates at <strong>the</strong><br />
mitotic spindle until late anaphase.<br />
APC/C function is regulated by (i) phosphorylation, <strong>and</strong> (ii) association of activator proteins such as<br />
Cdc 20 <strong>and</strong> its homologue Hct1 (homologue of Cdc20/Cdh1), which modulate <strong>the</strong> affinity of APC/C for<br />
different substrates [Peters, 2002]. The activation of <strong>the</strong> SCP inhibits <strong>the</strong> ubiquitin-dependent<br />
proteolysis of securin by <strong>the</strong> APC Cdc20 (APC/C activated by Cdc20) [Hwang et al., 1998].<br />
<strong>10</strong>.2.6 Spindle Checkpoint (SCP)
The activity of <strong>the</strong> APC is controlled by <strong>the</strong> spindle checkpoint, a mechanochemical surveillance<br />
mechanism shared by most eukaryotic cells, that prevents sister chromatid separation when spindles<br />
are damaged or chromosomes fail to form spindle attachments [Amon, 1999; Nasmyth, 2002; Lew,<br />
2003; Lew & Burke, 2003; Gillett et al., 2004; Tan et al., 2005]. The kinetochore of a single lagging<br />
chromosome emits a signal capable of blocking separation of all sister pairs. It also blocks any fur<strong>the</strong>r<br />
cell cycle progression.<br />
Figure <strong>10</strong>-17: The kinetochore. (Reproduced from Tan et al., 2005).<br />
In yeast, <strong>the</strong> kinetochore (Figure <strong>10</strong>-17) is composed of protein assemblies that can be broadly<br />
classified as inner, central or outer kinetochore complexes [Tan et al., 2005, Ciferri et al., 2007]. The<br />
outer kinetochore complex DAM1, composed of Duo1 <strong>and</strong> Mps1 (monopolar spindle 1-interacting<br />
complex), plays a crucial role in mediating <strong>the</strong> kinetochore–microtubular connection, <strong>and</strong> is regulated<br />
through phosphorylation by <strong>the</strong> Ipl1p/Aurora B kinase. The central complex contains <strong>the</strong> IPL1, CFT19,<br />
NDC80, <strong>and</strong> MWT1 complexes which are associated with both microtubules via <strong>the</strong> DAM1 complex<br />
<strong>and</strong> kinetochores via <strong>the</strong> inner complex, <strong>the</strong> most critical complex is CBF3 (centromere binding factor<br />
3). CBF3 consists of <strong>the</strong> essential proteins Ndc<strong>10</strong>, Cep3, Cft13, <strong>and</strong> Skp1, as well as a number of<br />
chromatin-specific proteins, which are required to build up a kinetochore at each centromere. The IPL1<br />
complex responds to <strong>the</strong> lack of tension in monotelic attachments, <strong>and</strong> acts to resolve <strong>the</strong>se<br />
inappropriate attachments, probably through its substrates. In <strong>the</strong> absence of tension, Ipl1 causes an<br />
increased turnover of kinetochore–microtubule connections, perhaps by influencing <strong>the</strong> Ndc80–DAM1<br />
interaction. Experiments in budding yeast have demonstrated that <strong>the</strong> tension resulting from <strong>the</strong><br />
physical connection between bi-oriented kinetochores, <strong>and</strong> <strong>the</strong> activity of Ipl1 (ra<strong>the</strong>r than any specific<br />
chromosomal architecture or kinetochore geometry) is sufficient for <strong>the</strong> proper alignment of sister<br />
chromatids [Dewar et al., 2004].<br />
The process of APC control is triggered by <strong>the</strong> recruitment of a complex to <strong>the</strong> SCP (Figure <strong>10</strong>-18),<br />
which contains <strong>the</strong> proteins Mad1p, Mad2p, Mad3p, <strong>and</strong> <strong>the</strong> protein kinases Bub1p <strong>and</strong> Bub3p, of<br />
which Bub1p is activated through Cdc2. Bub3p binds to <strong>the</strong> activator Cdc20p of <strong>the</strong> APC/C complex<br />
<strong>and</strong> <strong>the</strong>reby blocks ubiquitination of both securin <strong>and</strong> cyclin B. In addition, protein kinase Msp1p is<br />
also required for spindle pole duplication as well as <strong>the</strong> subunit Ncd<strong>10</strong>p of <strong>the</strong> centromere binding<br />
complex CBF3. The Mad complex has been shown to be highly conserved among o<strong>the</strong>r eukaryotes.<br />
The functions of APC <strong>and</strong> fur<strong>the</strong>r factors involved in regulating mitotic spindle disassembly have been
intensely described [Schuyler et al., 2003; Pereira & Schiebel, 2003; Buvelot et al., 2003; Tan et al.,<br />
2005].<br />
A more detailed view of <strong>the</strong> function of <strong>the</strong> APC is presented in Figure <strong>10</strong>-19.<br />
Figure <strong>10</strong>-18: The spindle checkpoint.<br />
Figure <strong>10</strong>-19: Different states of <strong>the</strong> spindle checkpoint. (Reproduced from Tan et al., 2005.)<br />
(A) Recruitment: Mad1 <strong>and</strong> Mad2 form a tight complex that localizes to NPC (nuclear pore complex). Upon SCP engagement,<br />
subcomplexes of checkpoint proteins are recruited to <strong>the</strong> kinetochore. Cdc2-dependent Bub1 phosphorylation is required to<br />
activate <strong>the</strong> SCP after spindle damage. The kinases Mps1 <strong>and</strong> Bub1 act in concert <strong>and</strong> trigger <strong>the</strong> rapid recruitment of <strong>the</strong><br />
Mad1–Mad2 complex that disengages from <strong>the</strong> NPC upon SCP activation. Recruitment of SCP complexes presumably occurs<br />
via <strong>the</strong>ir direct or indirect interaction with kinetochore components <strong>and</strong> o<strong>the</strong>r checkpoint proteins. The DAM1 complex has been<br />
shown to bind Mps1, Mad1, Mad3, Bub1 <strong>and</strong> Bub2. Bub1 binds Skp1. CENP-I is also essential for <strong>the</strong> kinetochore association<br />
of Mad1 <strong>and</strong> Mad2. Mad1 also interacts with <strong>and</strong> forms a complex with <strong>the</strong> Bub1–Bub3 heterodimer in such a way that Bub3<br />
binds both Mad1 <strong>and</strong> Mad2. The domain of Bub1 required for binding Bub3 is <strong>the</strong> same domain that is required for localization<br />
of Bub1 to kinetochores, suggesting that, upon association with kinetochores, Bub3 dissociates from Bub1. Bub3 also forms a<br />
complex with Mad3 throughout <strong>the</strong> cell cycle <strong>and</strong> may target Mad3 to kinetochores. In addition to <strong>the</strong> SCP signalling molecules<br />
depicted in <strong>the</strong> Figure, Cdc20 <strong>and</strong> <strong>the</strong> APC/C are also recruited to kinetochores upon SCP activation. Red arrows show<br />
phosphorylation events.<br />
(B) Reorganization <strong>and</strong> turnover: red arrows show phosphorylation events <strong>and</strong> green arrows show protein turning over. SCP<br />
activation results in <strong>the</strong> hyperphosphorylation of Mad1 by Mps1 <strong>and</strong> perhaps also Bub1, <strong>and</strong> causes <strong>the</strong> formation of <strong>the</strong> Mad1–<br />
Bub1–Bub3 complex. The Mad1–Mad2 complex recruited to <strong>the</strong> kinetochore functions as a template for <strong>the</strong> rapid catalytic<br />
conversion <strong>and</strong> release of soluble Mad2 in an APC Cdc20 -inhibitory form, labelled in purple. Mad3/BubR1 <strong>and</strong> Bub3 are also<br />
turned over at kinetochores <strong>and</strong> released, although it is not known if <strong>the</strong>y are modified in <strong>the</strong> process. The APC Cdc20 -inhibitory<br />
form of Mad2 is transferred to <strong>the</strong> Bub3–Cdc20–Mad3/BubR1 complex, which is a potent inhibitor of APC/C.<br />
(C) Inhibition of anaphase: <strong>the</strong> SCP deploys various strategies to inhibit anaphase onset. Targeting of substrates by APC Cdc20 is<br />
inhibited independently <strong>and</strong> collaboratively by <strong>the</strong> various Mad–Bub complexes. In in vitro assays, both BubR1 <strong>and</strong> Mad2 block<br />
<strong>the</strong> binding of Cdc20 to APC/C. Mitotic APC/C (with some APC/C subunits phosphorylated by Bub1 <strong>and</strong> Mps1) is more
susceptible to inhibition upon SCP activation as compared with APC/C from o<strong>the</strong>r stages of <strong>the</strong> cell cycle. It has also been<br />
shown that Cdk1–cyclin B-dependent inhibitory phosphorylation of Cdc20/Fizzy contributes to keeping APC/C inactive under<br />
conditions of SCP activation. Fur<strong>the</strong>rmore, SCP activation triggers Cdc20 degradation <strong>and</strong> keeps <strong>the</strong> level of Cdc20 low until all<br />
chromosomes have bi-oriented. Restoration of attachment/tension at kinetochores results in <strong>the</strong> dynein-dependent depletion of<br />
SCP proteins by transport away from kinetochores towards <strong>the</strong> poles along spindle MTs. Regulated translocation of Mad1 <strong>and</strong><br />
Mad2 away from kinetochores is believed to make <strong>the</strong> depletion less reversible. Bub1 persists longer than Mad1 <strong>and</strong> Mad2 at<br />
kinetochores, <strong>and</strong> <strong>the</strong> consequent physical separation of components of <strong>the</strong> SCP catalytic scaffold turns off <strong>the</strong> SCP signal,<br />
even though Mad2 continues to turn over at <strong>the</strong> spindle poles.<br />
<strong>10</strong>.3 Sexual Reproduction<br />
Though vegetative growth is <strong>the</strong> major way of yeast reproduction, sexual reproduction is an<br />
alternative when nutrient supplies fall short. The latter process involves <strong>the</strong> conjugation of cells of<br />
opposite mating type [Shimoda, 2004]. A heterokaryon with a diploid set of chromosomes is formed,<br />
which is capable of reproduction by budding. Under starvation conditions, meiosis is induced which<br />
leads to sporulation <strong>and</strong> finally to <strong>the</strong> propagation of four haploid spores that segregate 2:2 (cf. chapter<br />
1).<br />
<strong>10</strong>.3.1 Mating Types <strong>and</strong> Mating<br />
<strong>Cell</strong>s of opposite mating type (a <strong>and</strong> α) synchronize each o<strong>the</strong>rs' cell cycles at START in response to<br />
<strong>the</strong>ir mating factors. Once cells progress through START, <strong>the</strong>y are unable to mate until <strong>the</strong> next cell<br />
cycle. Conjugation occurs by surface contact of specialized projections ('schmoo' formation) on mating<br />
cells followed by plasma membrane fusion. The mechanism of <strong>the</strong> mating response will be discussed<br />
in more detail in chapter 13. After spore germination, haploid cells have <strong>the</strong> capability of undergoing<br />
mating type switch which maximizes <strong>the</strong> potential of diploidy. Wild-type strains are homothallic <strong>and</strong> a/α<br />
diploids represent <strong>the</strong> usual vegetative state of this yeast. Industrial (brewing) yeast strains are<br />
generally polyploid <strong>and</strong> do not undergo a sexual life cycle.<br />
<strong>10</strong>.3.2 Meiosis <strong>and</strong> Sporulation<br />
Figure <strong>10</strong>-20: Meiosis in yeast.
Meiosis is a variation on <strong>the</strong> <strong>the</strong>me of mitosis. It produces haploid gametes that contain recombinant<br />
chromosomes, parts of which are derived from one parent <strong>and</strong> parts of which are derived from <strong>the</strong><br />
o<strong>the</strong>r parent. As in <strong>the</strong> case of mitosis, meiosis starts with a round of DNA replication in diploid cells,<br />
which produces connected sister chromatids (Figure <strong>10</strong>-20). Recombination between homologous<br />
chromatids brings homologues toge<strong>the</strong>r.<br />
During <strong>the</strong> first meiotic division (meiosis I), sister kinetochores are treated as a single unit, <strong>and</strong><br />
homologous kinetochore pairs are attached to <strong>and</strong> are pulled towards opposite spindle poles.<br />
However, as long as at least one reciprocal exchange has occurred, cohesion between sister<br />
chromatid arms will oppose disjunction of homologues <strong>and</strong> <strong>the</strong>ir segregation to opposite poles of <strong>the</strong><br />
cell. Thus, loss of sister-chromatid cohesion along chromosome arms is essential for chromosome<br />
segregation during meiosis I. Meanwhile, however, cohesion between sister centromeres persists so<br />
that it can be later used to align sisters on <strong>the</strong> meiosis II metaphase plate. The difference in timing of<br />
sister chromatid cohesion loss is <strong>the</strong>refore a crucial aspect. Similar cohesion proteins as required for<br />
mitosis seem to work in meiosis.<br />
Meiosis produces haploid gametes (spores in yeast) from diploid cells in two stages that in many ways<br />
resemble mitosis [Murakami & Nurse, 2000]. During meiosis, two rounds of chromosome<br />
segregation occur after a single round of DNA replication, producing haploid progeny from diploid<br />
progenitors. Chromosome behaviour during meiosis I, however, is distinct from that in mitosis: (i)<br />
crossovers between maternal <strong>and</strong> paternal sister chromatids (detected cytologically as chiasmata)<br />
bind replicated maternal <strong>and</strong> paternal chromosomes toge<strong>the</strong>r; (ii) sister kinetochores attach to<br />
microtubules from <strong>the</strong> same pole (mono-polar orientation), causing maternal <strong>and</strong> paternal centromere<br />
pairs (<strong>and</strong> not sister chromatids) to be separated <strong>and</strong> segregated to <strong>the</strong> opposite sides of a cell; (iii)<br />
sister chromatid cohesion near centromeres must be protected throughout anaphase when cohesion<br />
along chromosome arms is beginning to be destroyed, a process which is regulated by Polo-like<br />
kinases such as Cdc5p. Moreover, Cdc5 is required both for <strong>the</strong> formation of chiasmata <strong>and</strong> for<br />
cosegregation of sister centromeres at meiosis I [Clyne et al., 2003]. The residual cohesion around<br />
centromeres plays an essential role at <strong>the</strong> second meiotic division, when spindle microtubules from<br />
opposite poles attach to sister chromatids. What protects centromeric cohesin from <strong>the</strong> prophase<br />
pathway? Recent studies have identified novel <strong>and</strong> conserved meiosis-specific kinetochore proteins,<br />
such as monopolin <strong>and</strong> shugoshin, which take this role of guardians [Watanabe, 2004; 2005]. The<br />
yeast kinetochore associated protein, Mam1 (Monopolin), is essential for monopolar attachment [Toth<br />
et al., 2000], while <strong>the</strong> meiosis-specific cohesin, Rec8, is essential for maintaining cohesion between<br />
sister centromeres but not for monopolar attachment. The yeast shugoshin, Sgo1p, prevents removal<br />
of meiotic cohesin complexes from centromeres at <strong>the</strong> first meiotic division, <strong>and</strong> it appears that<br />
shugoshin prevents phosphorylation of cohesin's Scc3-SA2 subunit at centromeres during mitosis.<br />
This ensures that cohesin persists at centromeres until activation of separase causes cleavage of its<br />
alpha kleisin subunit [Rabitsch et al., 2003; Katis et al., 2004a; 2004b; McGuinness et al., 2005]. In<br />
yeast, shugoshin also has a crucial role in sensing <strong>the</strong> loss of tension at kinetochores <strong>and</strong> in<br />
generating <strong>the</strong> spindle checkpoint signal [Watanabe, 2005].<br />
Sporulation, which in yeast is induced at (nitrogen) starvation, is regulated by a specialized MAP<br />
kinase signalling pathway (see chapter 13). In many aspects, starvation is similar to o<strong>the</strong>r stress<br />
responses in yeast. Spore formation requires <strong>the</strong> activation of a large number of genes, several of<br />
which are involved in <strong>the</strong> biosyn<strong>the</strong>sis of spore walls.
References<br />
Amon, A. (1999) The spindle checkpoint. Curr. Opin. Genet.Dev. 9, 69-75.<br />
Anderson, D.E., Losada, A., Erickson, H.P., Hirano, T. (2002) Condensin <strong>and</strong> cohesin display different arm<br />
conformations with characteristic hinge angles. J. <strong>Cell</strong> Biol. 156, 419-424.<br />
Arumugam, P., Nishino, T., Haering, C.H., Gruber, S., Nasmyth, K. (2006) Cohesin's ATPase activity is stimulated<br />
by <strong>the</strong> C-terminal Winged-Helix domain of its kleisin subunit. Curr Biol. 16, 1998-2008.<br />
Barral, Y., Mermall, V., Mooseker, M.S., Snyder, M. Compartmentalization of <strong>the</strong> cell cortex by septins is required<br />
for maintenance of cell polarity in yeast. Mol. <strong>Cell</strong>. 5 (2000) 841-51<br />
Barral, Y., Parra, M., Bidlingmaier, S., Snyder, M. (1999) Nim1-related kinases coordinate cell cycle progression<br />
with <strong>the</strong> organization of <strong>the</strong> peripheral cytoskeleton in yeast. Genes Dev. 13, 176–187.<br />
Barrett, J.G., Manning, B.D., Snyder, M. (2000) The Kar3p kinesin-related protein forms a novel heterodimeric<br />
structure with its associated protein Cik1p. Mol Biol <strong>Cell</strong>. 11, 2373-2385.<br />
Beach, D., Durkacz, B. et al. (1982) Functionally homologous cell cycle control genes in budding <strong>and</strong> fission<br />
yeast. Nature 300, 706–709.<br />
Bell, S.P., Mitchell, J., Leber, J., Kobayashi, R., Stillman, B. (1995) The multidomain structure of Orc1p reveals<br />
similarity to regulators of DNA replication <strong>and</strong> transcriptional silencing. <strong>Cell</strong> 83, 563-568.<br />
Bidlingmaier, S., Weiss, E.L., Seidel, C., Drubin, D.G., Snyder, M. (2001) The Cbk1p pathway is important for<br />
polarized cell growth <strong>and</strong> cell separation in Saccharomyces cerevisiae. Mol. <strong>Cell</strong>. Biol. 21, 2449-2462.<br />
Biggins, S., Murray, A.W. Sister chromatid cohesion in mitosis. Curr. Opin. Genet. Dev. 9 (1999) 230-236.<br />
Blackburn, E.H., Greider, C.W., Szostak, J.W. (2006) Telomeres <strong>and</strong> telomerase: <strong>the</strong> path from maize,<br />
Tetrahymena <strong>and</strong> yeast to human cancer <strong>and</strong> aging. Nat Med. 12, 1133-1138.<br />
Boule, J.P., Zakian, V.A. (2006) Roles of Pif1-like helicases in <strong>the</strong> maintenance of genomic stability. Nucleic Acids<br />
Res. 34, 4147–4153.<br />
Bouquin, N., Barral, Y., Courbeyrette, R., Blondel, M., Snyder, M., Mann, C. Regulation of cytokinesis by <strong>the</strong> Elm1<br />
protein kinase in Saccharomyces cerevisiae. J. <strong>Cell</strong> Sci. 113 (2000) 1435-1445.<br />
Brigati, C., Kurtz, S., Balderes, D., Vidali, G., Shore, D. (1993) An essential yeast gene encoding a TTAGGG<br />
repeat-binding protein. Mol <strong>Cell</strong> Biol. 13, 1306-1314.<br />
Buvelot, S., Tatsutani, S.Y., Vermaak, D., Biggins, S. (2003) The budding yeast Ipl1/Aurora protein kinase<br />
regulates mitotic spindle disassembly. J. <strong>Cell</strong> Biol. 160, 329-339.<br />
Camobel, J.L., Cohen-Fix,O. Chromosome cohesion: ring around <strong>the</strong> sisters? Trends Biochem. Sci. 27 (2002)<br />
492-495.<br />
Casamayor, A., Snyder, M. (2003) Molecular Dissection of a <strong>Yeast</strong> Septin: Distinct Domains Are Required for<br />
Septin Interaction, Localization, <strong>and</strong> Function. Mol. <strong>Cell</strong> Biol. 23, 2762–2777.<br />
Cheeseman, I.M., Desai, A. (2004) <strong>Cell</strong> division: AAAtacking <strong>the</strong> mitotic spindle. Curr Biol. 14, R70-72. Review.<br />
Ciosk, R., Zachariae, W., Michaelis, C., Shevchenko, A., Mann, M., Nasmyth, K. (1998) An ESP1/PDS1 complex<br />
regulates loss of sister chromatid cohesion at <strong>the</strong> metaphase to anaphase transition in yeast. <strong>Cell</strong> 93, <strong>10</strong>67–<br />
<strong>10</strong>76.<br />
Clyne, R.K., Katis, V.L., Jessop, L., Benjamin, K.R., Herskowitz, I., Lichten, M., Nasmyth, K. (2003) Polo-like<br />
kinase Cdc5 promotes chiasmata formation <strong>and</strong> cosegregation of sister centromeres at meiosis I. Nat <strong>Cell</strong><br />
Biol. 5, 480-485.<br />
Coelho, P.S., Kumar, A., Snyder, M. Genome-wide mutant collections: toolboxes for functional genomics. Curr.<br />
Opin. Microbiol. 3 (2000) 309-315.<br />
Cohen-Fix, O., Koshl<strong>and</strong>, D. (1997) The anaphase inhibitor of Saccharomyces cerevisiae Pds1p is a target of <strong>the</strong><br />
DNA damage checkpoint pathway. Proc Natl Acad Sci U S A 94, 14361-1436.<br />
Cohen-Fix, O., Peters, J.M., Kirschner, M.W., Koshl<strong>and</strong>, D. (1996) Anaphase initiation in Saccharomyces<br />
cerevisiae is controlled by <strong>the</strong> APC-dependent degradation of <strong>the</strong> anaphase inhibitor Pds1p. Genes Dev <strong>10</strong>,<br />
3081-3093.<br />
Cohn, M., Blackburn, E.H. (1995) Telomerase in yeast. Science 269, 396-400.<br />
Cross, R.A. Molecular motors: dyneins gearbox. Cur. Biol. 14 (2004) R355-356.<br />
Dewar, H., Tanaka, K., Nasmyth, K., Tanaka, T.U. (2004) Tension between two kinetochores suffices for <strong>the</strong>ir biorientation<br />
on <strong>the</strong> mitotic spindle. Nature 428, 93-97.<br />
Diffley, J.F., Cocker, J.H. (1992) Protein-DNA interactions at a yeast replication origin. Nature 357, 169-172.<br />
Dirick, L., Bohm, T., Nasmyth, K. (1995) Roles <strong>and</strong> regulation of Cln-Cdc28 kinases at <strong>the</strong> start of <strong>the</strong> cell cycle of<br />
Saccharomyces cerevisiae. EMBO J.14, 4803-4813.<br />
Donaldson, A.D., Blow, J.J. The regulation of replication origin activation. Curr. Opin. Genet. Dev. 9 (1999) 62-<br />
68.<br />
Du, Y.C., Stillman, B. (2002) Yph1p, an ORC-interacting protein: potential links between cell proliferation control,<br />
DNA replication, <strong>and</strong> ribosome biogenesis. <strong>Cell</strong> <strong>10</strong>9, 835-848.<br />
Evans, T., Hunt, T., Youngblom, J. (1982) On <strong>the</strong> role of maternal mRNA in sea urchins: studies of a protein<br />
which appears to be destroyed at a particular point in each cell division cycle. Biol. Bull. 163, 372.<br />
Evans, T., Rosenthal, E.T., Youngbloom, J., Distel, D., Hunt, T. (1983). Cyclin: a protein specified by maternal<br />
mRNA in sea urchin eggs that is destroyed at each cleavage division. <strong>Cell</strong> 33, 389–396.<br />
Fisher, T.S., Taggart, A.K.P. , Zakian,V.A. (2004) <strong>Cell</strong> cycle-dependent regulation of yeast telomerase by Ku. Nat.<br />
Struct. Mol. Biol., 11, 1198–1205.<br />
Fleming, W. (1879) Archiv f. mikroskop. Anatomie 18, 151-259. Koshl<strong>and</strong>, D., Hartwell, L.H.(1987) The structure<br />
of sister minichromosome DNA before anaphase in Saccharomyces cerevisiae. Science 238, 1713-1736.
Foss, M., McNally, F.J., Laurenson, P., Rine, J. (1993) Origin recognition complex (ORC) in transcriptional<br />
silencing <strong>and</strong> DNA replication in S. cerevisiae. Science 262, 1838-1844.<br />
Futcher, B. Microarrays <strong>and</strong> cell cycle transcription in yeast. Cur. Opin. <strong>Cell</strong> Biol. 12 (2000) 7<strong>10</strong>–715.<br />
Gillett, E. S., Espelin, C. W., Sorger, P. K. (2004) Spindle checkpoint proteins <strong>and</strong> chromosome-microtubule<br />
attachment in budding yeast. J. <strong>Cell</strong> Biol. 164, 535–546.<br />
Gilson, E., Geli, V. (2007) How telomeres are replicated. Nat Rev Mol <strong>Cell</strong> Biol 8, 825-838.<br />
Glotzer, M, Murray, A.W., Kirschner, M.W. (1991). Cyclin is degraded by <strong>the</strong> ubiquitin pathway. Nature 349: 132–<br />
138.<br />
Gould, K. L., Nurse, P. (1989) Tyrosine phosphorylation of <strong>the</strong> fission yeast cdc2+ protein kinase regulates entry<br />
into mitosis. Nature 342, 39–45.<br />
Gruber, S., Arumugam, P., Katou, Y., Kuglitsch, D., Helmhart, W., Shirahige, K., Nasmyth, K. (2006) Evidence<br />
that loading of cohesin onto chromosomes involves opening of its SMC hinge. <strong>Cell</strong> 127, 523-537.<br />
Guacci, V., Koshl<strong>and</strong>, D. Strunnikov, A. (1997) A direct link between sister chromatid cohesion <strong>and</strong> chromosome<br />
condensation revealed through <strong>the</strong> analysis of MCD1 in S. cerevisiae. <strong>Cell</strong> 91, 47-57.<br />
Haering, C.H., Lowe, J., Hochwagen, A., Nasmyth, K. (2002) Molecular architecture of SMC proteins <strong>and</strong> <strong>the</strong><br />
yeast cohesin complex. Mol. <strong>Cell</strong> 9, 773-788.<br />
Hardy, C.F., Sussel, L., Shore, D. (1992) A RAP1-interacting protein involved in transcriptional silencing <strong>and</strong><br />
telomere length regulation. Genes Dev. 6, 801-814.<br />
Hartwell, L. H. (1971b) Genetic control of <strong>the</strong> cell division cycle in yeast. IV. Genes controlling bud emergence<br />
<strong>and</strong> cytokinesis. Exp. <strong>Cell</strong> Res. 69, 265–276.<br />
Hartwell, L. H., Hutchison, H. T., Holl<strong>and</strong>, T. M., McLaughlin, C. S. (1970a) The effect of cycloheximide upon<br />
polyribosome stability in two yeast mutants defective respectively in <strong>the</strong> initiation of polypeptide chains <strong>and</strong><br />
in messenger RNA syn<strong>the</strong>sis. Mol. Gen. Genet. <strong>10</strong>6, 347-361.<br />
Hartwell, L. H., Mortimer, R.K., Culotti, J., Culotti, M. (1974) Genetic control of <strong>the</strong> cell division cycle in yeast. V.<br />
Genetic analysis of cdc mutants. Genetics 74, 267–286.<br />
Hartwell, L., Weinert, T. (1989) Checkpoints: controls that ensure <strong>the</strong> order of cell cycle events. Science 246,<br />
629–634.<br />
Hartwell, L.H. (1967) Macromolecule syn<strong>the</strong>sis in temperature-sensitive mutants of yeast. J Bacteriol. 93, 1662-<br />
1670.<br />
Hartwell, L.H. (1971a) Genetic control of <strong>the</strong> cell division cycle in yeast. II. Genes controlling DNA replication <strong>and</strong><br />
its initiation. J Mol Biol. 59, 183-194.<br />
Hartwell, L.H. (1973) Synthronization of haploid yeast cell cycles, a prelude to conjugation. Exp. <strong>Cell</strong> Res.<br />
76,111–117.<br />
Hartwell, L.H. (1974) Saccharomyces cerevisiae cell cycle. Bacteriol Rev. 38, 164-198. Review.<br />
Hartwell, L.H. (2002) Nobel Lecture. <strong>Yeast</strong> <strong>and</strong> cancer. Biosci Rep. 22, 373-394.<br />
Heun, P., Laroche, T., Shimada, K., Furrer, P., Gasser, S.M. Chromosome dynamics in <strong>the</strong> yeast interphase<br />
nucleus. Science 294 (2001) 2181-2186.<br />
Hirano, T (2000) Chromosome cohesion, condensation, <strong>and</strong> separation. Ann. Rev. Biochem. 69, 115 -144.<br />
Holloway, S.L., Glotzer, M., King, R.W., Murray, A.W. (1993) Anaphase is initiated by proteolysis ra<strong>the</strong>r than by<br />
<strong>the</strong> inactivation of maturation-promoting factor. <strong>Cell</strong> 73, 1393-1402.<br />
Hunt, T. (2001) Protein Syn<strong>the</strong>sis, Proteolysis <strong>and</strong> <strong>Cell</strong> <strong>Cycle</strong> Transitions. Nobel Lecture, 2001.<br />
Hwang, L. H., Lau, L. F., Smith, D. L., Mistrot, C. A., Hardwick, K. G., Hwang, E. S., Amon, A., Murray, A. W.<br />
(1998) Budding yeast Cdc20: a target of <strong>the</strong> spindle checkpoint. Science 279, <strong>10</strong>41–<strong>10</strong>44.<br />
Irniger, S., Piatti, S., Michaelis, C., Nasmyth, K. (1995) Genes involved in sister chromatid separation are needed<br />
for B-type cyclin proteolysis in budding yeast. <strong>Cell</strong> 81, 269-278.<br />
Ivanov, D., Nasmyth, K. (2005) A topological interaction between cohesin rings <strong>and</strong> a circular minichromosome.<br />
<strong>Cell</strong> 122, 849-860.<br />
Iyer, V.R., Horak, C.E., Scafe, C.S., Botstein, D., Snyder, M., Brown, P.O. Genomic binding sites of <strong>the</strong> yeast cellcycle<br />
transcription factors SBF <strong>and</strong> MBF. Nature 409 (2001) 533-538.<br />
Jaspersen, S.L., Winey, M. (2004) The budding yeast spindle pole body: Structure, Duplication, <strong>and</strong> Function.<br />
Annu. Rev. <strong>Cell</strong> Dev. Biol. 20, 1–28.<br />
Katis V.L., Matos, J., Mori, S., Shirahige, K., Zachariae, W., Nasmyth, K. (2004a) Spo13 facilitates monopolin<br />
recruitment to kinetochores <strong>and</strong> regulates maintenance of centromeric cohesion during yeast meiosis. Curr<br />
Biol. 14, 2183-2196.<br />
Katis, V.L., Galova, M., Rabitsch, K.P., Gregan, J., Nasmyth, K. (2004b) Maintenance of cohesin at centromeres<br />
after meiosis I in budding yeast requires a kinetochore-associated protein related to MEI-S332. Curr Biol. 14,<br />
560-572.<br />
King, R.W. Peters, J.M., Tugendreich, S., Rolfe, M., Hieter, P., Kirschner, M.W. (1995) A 20S complex containing<br />
CDC27 <strong>and</strong> CDC16 catalyzes <strong>the</strong> mitosis-specific conjugation of ubiquitin to cyclin B. <strong>Cell</strong> 81, 269-278.<br />
Kurtz, S., Shore, D. (1991) RAP1 protein activates <strong>and</strong> silences transcription of mating-type genes in yeast.<br />
Genes Dev. 5, 616-628.<br />
Lauren M., DeSalle, L.M., Pagano, M. Regulation of <strong>the</strong> G1 to S transition by <strong>the</strong> ubiquitin pathway. FEBS Lett.<br />
490 (2001) 179-189.<br />
Lavoie, B.D., Hogan, E., Koshl<strong>and</strong>, D. (2002) In vivo dissection of <strong>the</strong> chromosome condensation machinery:<br />
reversibility of condensation distinguishes contributions of condensin <strong>and</strong> cohesin. J <strong>Cell</strong> Biol. 156, 805-815.<br />
Lee, M. G., Nurse, P. (1987) Complementation used to clone a human homologue of <strong>the</strong> fission yeast cell cycle<br />
control gene cdc2. Nature 327, 31–35.<br />
Lew, D. J., Burke, D. J. (2003) The spindle assembly <strong>and</strong> spindle position checkpoints. Annu. Rev. Genet. 37,<br />
251–282.
Lew, D.J. (2003) The morphogenesis checkpoint: how yeast cells watch <strong>the</strong>ir figures. Curr. Opin. <strong>Cell</strong> Biol. 15,<br />
648–653.<br />
Li, R., Yu, D.S., Tanaka, M., Zheng, L., Berger, S.L., Stillman, B. (1998) Activation of chromosomal DNA<br />
replication in Saccharomyces cerevisiae by acidic transcriptional activation domains. Mol <strong>Cell</strong> Biol. 18, 1296-<br />
1302.<br />
Liang, C., Weinreich, M., Stillman, B. (1995) ORC <strong>and</strong> Cdc6p interact <strong>and</strong> determine <strong>the</strong> frequency of initiation of<br />
DNA replication in <strong>the</strong> genome. <strong>Cell</strong> 81, 667-676.<br />
Loo, S., Fox, C.A., Rine, J., Kobayashi, R., Stillman, B., Bell, S. (1995) The origin recognition complex in<br />
silencing, cell cycle progression, <strong>and</strong> DNA replication. Mol Biol <strong>Cell</strong> 6, 741-756.<br />
Losada, A., Hirano, M., Hirano, T. (1998) Identification of Xenopus SMC protein complexes required for sister<br />
chromatid cohesion. Genes Dev. 12, 1986-1997.<br />
Lundblad, V. (2003) Telomere replication: an Est fest. Curr. Biol., 13, R439–R441.<br />
Lustig, A.J., Kurtz, S., Shore, D. (1990) Involvement of <strong>the</strong> silencer <strong>and</strong> UAS binding protein RAP1 in regulation of<br />
telomere length. Science 250, 549-553.<br />
Mala, J., Nurse, P. Trends <strong>Cell</strong> Biol. 8 (1998) 163-169.<br />
Manning, B.D., Barrett, J.G., Wallace, J.A., Granok, H., Snyder, M. Differential regulation of <strong>the</strong> Kar3p kinesinrelated<br />
protein by two associated proteins, Cik1p <strong>and</strong> Vik1p. J. <strong>Cell</strong> Biol. 22 (1999) 1219-1233.<br />
Manning, B.D., Barrett, J.G., Wallace, J.A., Granok, H., Snyder, M. (1999) Differential regulation of <strong>the</strong> Kar3p<br />
kinesin-related protein by two associated proteins, Cik1p <strong>and</strong> Vik1p. J. <strong>Cell</strong> Biol. 22 , 1219-1233.<br />
Manning, B.D., Snyder, M. (2000) Drivers <strong>and</strong> passengers wanted! <strong>the</strong> role of kinesin-associated proteins. Trends<br />
<strong>Cell</strong>.Biol. <strong>10</strong>, 281-289.<br />
Manning, B.D., Snyder, M. Drivers <strong>and</strong> passengers wanted! <strong>the</strong> role of kinesin-associated proteins. Trends<br />
<strong>Cell</strong>.Biol. <strong>10</strong> (2000) 281-289.<br />
McGuinness, B.E., Hirota, T., Kudo, N.R., Peters, J.M., Nasmyth, K. (2005) Shugoshin prevents dissociation of<br />
cohesin from centromeres during mitosis in vertebrate cells. PLoS Biol. 3, e86.<br />
McLaughlin, C.S., Hartwell, L.H. (1969) Mutants of yeast defective in <strong>the</strong> initiation of protein biosyn<strong>the</strong>sis. Cold<br />
Spring Harb Symp Quant Biol. 34, 317-319.<br />
Michaelis, C., Ciosk, R., Nasmyth, K. (1997) Cohesins: chromosomal proteins that prevent premature separation<br />
of sister chromatids. <strong>Cell</strong> 91, 35-45.<br />
Micklem, G., Rowley, A., Harwood, J., Nasmyth, K., Diffley, J.F. (1993) <strong>Yeast</strong> origin recognition complex is<br />
involved in DNA replication <strong>and</strong> transcriptional silencing. Nature 366, 87-89.<br />
Mitchison, J.M. (1971) The biology of <strong>the</strong> cell cycle. Cambridge: Cambridge University Press.<br />
Moreno, S., Hayles, J., Nurse, P. (1989) Regulation of p34cdc2 protein kinase during mitosis. <strong>Cell</strong> 58, 361–372.<br />
Moretti, P., Freeman, K., Coodly, L., Shore, D. (1994) Evidence that a complex of SIR proteins interacts with <strong>the</strong><br />
silencer <strong>and</strong> telomere-binding protein RAP1. Genes Dev. 8, 2257-2269.<br />
Murakami, H., Nurse, P. (2000) DNA replication <strong>and</strong> damage checkpoints <strong>and</strong> meiotic cell cycle controls in <strong>the</strong><br />
fission <strong>and</strong> budding yeasts. Biochem. J. 349, 1-12. Review.<br />
Nasmyth, K. (2001) Disseminating <strong>the</strong> genome: joining, resolving, <strong>and</strong> separating sister chromatids during mitosis<br />
<strong>and</strong> meiosis. Ann. Rev. Genet. 35, 673 – 745.<br />
Nasmyth, K. Segregating sister genomes: <strong>the</strong> molecular biology of chromosome separation. Science 297 (2002)<br />
559-565.<br />
Nasmyth, K. Separating sister chromatids. Trends Biochem. Sci. 24 (1999) 98-<strong>10</strong>4.<br />
Nasmyth, K.A., Reed, S,I. (1980) Isolation of genes by complementation in yeast: molecular cloning of a cell-cycle<br />
gene. Proc Natl Acad Sci U S A 77, 2119-2123.<br />
Ni, L., Snyder, M. (2001) A genomic study of <strong>the</strong> bipolar bud site selection pattern in Saccharomyces cerevisiae.<br />
Mol. Biol. <strong>Cell</strong> 12, 2147-2170.<br />
Nurse, P. (1990) Universal control mechanism regulating onset of M-phase. Nature 344, 503–508.<br />
Nurse, P. (2000) A long twentieth century of <strong>the</strong> cell cycle <strong>and</strong> beyond. <strong>Cell</strong> <strong>10</strong>0, 71–78.<br />
Nurse, P., Bissett, Y. (1981) Gene required in G1 for commitment to cell cycle <strong>and</strong> in G2 for control of mitosis in<br />
fission yeast. Nature 292, 558–560.<br />
Nurse, P., Thuriaux, P. (1980) Regulatory genes controlling mitosis in <strong>the</strong> fission yeast Schizosaccharomyces<br />
pombe. Genetics 96, 627–637.<br />
Nurse, P., Thuriaux, P., Nasmyth, K. (1976) Genetic control of <strong>the</strong> cell division cycle in <strong>the</strong> fission yeast S. pombe.<br />
Mol. Gen. Genet. 146, 167-178.<br />
Nurse, P.M. (2001) Cyclin dependent kinases <strong>and</strong> cell cycle control. Nobel Lecture, December 9, 2001<br />
Ooi, S.L., Shoemaker, D.D., Boeke, J.D. A DNA microarray-based genetic screen for non-homologous endjoining<br />
mutants in Saccharomyces cerevisiae. Science 294 (2001) 2552-2556.<br />
Paulovich, A.G., Toczyski, D.P., Hartwell, L.H. (1997) When checkpoints fail. <strong>Cell</strong> 88, 315-321.<br />
Pereira, G., Schiebel, E. (2003) Separase regulates INCENPAurora B anaphase spindle function through Cdc14.<br />
Science 302, 2120-2124.<br />
Peters, J. M. (2002) The anaphase-promoting complex: proteolysis in mitosis <strong>and</strong> beyond. Mol. <strong>Cell</strong> 9, 931–943.<br />
Rabitsch, K.P., Petronczki, M., Javerzat, J.P., Genier, S., Chwalla, B., Schleiffer, A., Tanaka, T.U., Nasmyth, K.<br />
(2003) Kinetochore recruitment of two nucleolar proteins is required for homolog segregation in meiosis I.<br />
Dev <strong>Cell</strong>. 4, 535-548.<br />
Raghuraman, M.K., Winzeler, E.A., Collingwood, D., Hunt, S., Wodicka, L., Conway, A., Lockhart, D.J., Davis,<br />
R.W., Brewer, B.J., Fangman, W.L. (2001) Replication dynamics of <strong>the</strong> yeast genome. Science 294, 115-<br />
121.<br />
Rao, H., Stillman, B. (1995) The origin recognition complex interacts with a bipartite DNA binding site within yeast<br />
replicators. Proc Natl Acad Sci U S A. 92, 2224-2228.
Rao, H., Uhlmann, F., Nasmyth, K,, Varshavsky, A. (2001) Degradation of a cohesin subunit by <strong>the</strong> N-end rule<br />
pathway is essential for chromosome stability. Nature 4<strong>10</strong>, 955-959.<br />
Reid, R.J., Rothstein, R. (2004) Stay close to your sister. Mol <strong>Cell</strong>. 14, 418-420. Review<br />
Ricchetti, M., Fairhead, C., Dujon, B. Mitochondrial DNA repairs double-str<strong>and</strong> breaks in yeast chromosomes.<br />
Nature 402 (1999) 96-<strong>10</strong>0.<br />
Roemer, T., Vallier, L.G., Snyder, M. (1996) Selection of polarized growth sites in yeast. Trends <strong>Cell</strong> Biol. 6, 434-<br />
441.<br />
Schuyler, S.C., Liu, J.Y., Pellman, D. (2003) The molecular function of Ase1p: evidence for a MAP-dependent<br />
midzone-specific spindle matrix. Microtubule-associated proteins. J. <strong>Cell</strong> Biol. 160, 517-528.<br />
Segal, M., Bloom, K. (2001) Control of spindle polarity <strong>and</strong> orientation in S. cerevisiae. Trends <strong>Cell</strong> Biol. 11, 160-<br />
166.<br />
Sheu, Y.J., Barral, Y., Snyder, M.(2000) Polarized growth controls cell shape <strong>and</strong> bipolar bud site selection in<br />
Saccharomyces cerevisiae. Mol. <strong>Cell</strong>. Biol. 20, 5235-5247.<br />
Shimoda, C. (2004) Forespore membrane assembly in yeast: coordinating SPBs <strong>and</strong> membrane trafficking. J.<br />
<strong>Cell</strong> Sci. 17, 389-395.<br />
Shintomi, K., Hirano, T. (2007) How are cohesin rings opened <strong>and</strong> closed? Trends Biochem Sci. 32, 154-157.<br />
Shore, D. (1997) Telomerase <strong>and</strong> telomere-binding proteins: controlling <strong>the</strong> endgame. Trends Biochem Sci. 22,<br />
233-235. Review.<br />
Shore, D. (1998) <strong>Cell</strong>ular senescence: lessons from yeast for human aging? Curr Biol. 8, R192-195. Review.<br />
Shore, D. (2001) Telomeric chromatin: replicating <strong>and</strong> wrapping up chromosome ends. Curr Opin Genet Dev. 11,<br />
189-198. Review.<br />
Shore, D., Nasmyth, K. (1987) Purification <strong>and</strong> cloning of a DNA binding protein from yeast that binds to both<br />
silencer <strong>and</strong> activator elements. <strong>Cell</strong> 51, 721-732.<br />
Shore, D., Stillman, D.J., Br<strong>and</strong>, A.H., Nasmyth, K.A. (1987) Identification of silencer binding proteins from yeast:<br />
possible roles in SIR control <strong>and</strong> DNA replication. EMBO J. 6, 461-467.<br />
Skibbens, R.V., Corson, L.B., Koshl<strong>and</strong>, D., Hieter, P. (1999) Ctf7p is essential for sister chromatid cohesion <strong>and</strong><br />
links mitotic chromosome structure to <strong>the</strong> DNA replication machinery. Genes Dev. 13, 307-319.<br />
Smeal, T., Guarente, L. (1997) Mechanisms of cellular senescence. Curr Opin Genet Dev. 7, 281-287. Review.<br />
Stellwagen, A.E., Haimberger, Z.W., Veatch, J.R., Gottschling, D.E. (2003) Ku interacts with telomerase RNA to<br />
promote telomere addition at native <strong>and</strong> broken chromosome ends. Genes Dev., 17, 2384–2395.<br />
Stern, B., Nurse, P. (1996) A quantitative model for <strong>the</strong> cdc2 control of S phase <strong>and</strong> mitosis in fission yeast.<br />
Trends Genet 12, 345–350.<br />
Stillman, B. (2001) DNA replication. Genomic views of genome duplication. Science 294, 2301-2304.<br />
Stillman, B. (2005) Origin recognition <strong>and</strong> <strong>the</strong> chromosome cycle. FEBS Lett. 579, 877-884. Review.<br />
Stillman, B. DNA replication. Genomic views of genome duplication. Science 294 (2001) 2301-2304.<br />
Sudakin, V., Ganoth, D., Dahan, A., Heller, H., Hershko, J., Luca, F.C., Ruderman, J.V., Hershko, A. (1995) The<br />
cyclosome, a large complex containing cyclin-selective ubiquitin ligase activity, targets cyclins for destruction<br />
at <strong>the</strong> end of mitosis. Mol. Biol. <strong>Cell</strong> 6, 185-198.<br />
Surana, U., Amon, A., Dowzer, C., McGrew, J., Byers, B., Nasmyth, K. (1993) Destruction of <strong>the</strong> CDC28/CLB<br />
mitotic kinase is not required for <strong>the</strong> metaphase to anaphase transition in budding yeast. EMBO J. 12, 1969-<br />
1978.<br />
Swaroop, M., Wang, Y., Miller, P., Duan, H., Jatkoe, T., Madore, S.J., Sun, Y. <strong>Yeast</strong> homolog of human<br />
SAG/ROC2/Rbx2/Hrt2 is essential for cell growth, but not for germination: chip profiling implicates its role in<br />
cell cycle regulation. Oncogene 19 (2000) 2855-2866.<br />
Swaroop, M., Wang, Y., Miller, P., Duan, H., Jatkoe, T., Madore, S.J., Sun, Y. (2000) <strong>Yeast</strong> homolog of human<br />
SAG/ROC2/Rbx2/Hrt2 is essential for cell growth, but not for germination: chip profiling implicates its role in<br />
cell cycle regulation. Oncogene 19, 2855-2866.<br />
Taddei, A., Gasser, S.M. (2004) Multiple pathways for telomere te<strong>the</strong>ring: functional implications of subnuclear<br />
position for heterochromatin formation. Biochim. Biophys. Acta 1677, 120–128. Review.<br />
Taggart, A.K., Zakian, V.A. (2003) Telomerase: What are <strong>the</strong> EST proteins doing? Curr. Opin. <strong>Cell</strong> Biol., 15, 275–<br />
280.<br />
Taggart, A.K.P., Teng, S.C., Zakian, V.A. (2002) Est1p as a cell cycle-regulated activator of telomere-bound<br />
telomerase. Science, 297, <strong>10</strong>23–<strong>10</strong>26.<br />
Tan, A.L.C., Rida, P.G.C., Surana, U. (2005) Essential tension <strong>and</strong> constructive destruction: <strong>the</strong> spindle<br />
checkpoint <strong>and</strong> its regulatory links with mitotic exit. Biochem. J. 386, 1–13. Review.<br />
Teixeira, M.T.,Gilson, E. (2005) Telomere maintenance, function <strong>and</strong> evolution: <strong>the</strong> yeast paradigm. Chromo Res<br />
13, 535-548.<br />
Torres-Rosell, J., Machin, F., Aragon, L. (2005) Smc5-Smc6 complex preserves nucleolar integrity in S.<br />
cerevisiae. <strong>Cell</strong> <strong>Cycle</strong> 4, 868-872.<br />
Toth, A., Ciosk, R., Uhlmann, F., Galova, M., Schleiffer, A., Nasmyth, K. (1999) <strong>Yeast</strong> cohesin complex requires a<br />
conserved protein, Eco1p(Ctf7), to establish cohesion between sister chromatids during DNA replication.<br />
Genes Dev. 13, 320-333.<br />
Tóth, A., Rabitsch, K.P., Gálová, M., Schleiffer, A., Buonomo, S.B., Nasmyth, K. (2000) Functional genomics<br />
identifies monopolin: a kinetochore protein required for segregation of homologs during meiosis i. <strong>Cell</strong> <strong>10</strong>3,<br />
1155-1168.<br />
Uhlmann, F. The mechanism of sister chromatid cohesion. Exp. <strong>Cell</strong> Res. 296 (2004) 80-85.<br />
Uhlmann, F., Lottspeich, F., Nasmyth, K. (1999) Sister-chromatid separation at anaphase onset is promoted by<br />
cleavage of <strong>the</strong> cohesin subunit Scc1. Nature 400, 37–42.
Vogel, J., Drapkin, B., Oomen, J., Beach, D., Bloom, K., Snyder, M. Phosphorylation of gamma-tubulin regulates<br />
microtubule organization in budding yeast. Dev. <strong>Cell</strong>. 1 (2001) 621-631.<br />
Vogel, J., Snyder, M. (2000) Gamma-tubulin of budding yeast. Curr. Top. Dev. Biol. 49, 75-<strong>10</strong>4.<br />
Wang, B.D., Eyre, D., Basrai, M., Lichten, M., Strunnikov, A. (2005) Condensin binding at distinct <strong>and</strong> specific<br />
chromosomal sites in <strong>the</strong> Saccharomyces cerevisiae genome. Mol <strong>Cell</strong> Biol. 25, 7216-7225.<br />
Watanabe, Y. (2004) Modifying sister chromatid cohesion for meiosis. J <strong>Cell</strong> Sci. 117, 4017-4023.<br />
Watanabe, Y. (2005) Shugoshin: guardian spirit at <strong>the</strong> centromere. Curr Opin <strong>Cell</strong> Biol.17, 590-595.<br />
Weinreich, M., Liang, C., Chen, H.H., Stillman, B. (2001) Binding of cyclin-dependent kinases to ORC <strong>and</strong> Cdc6p<br />
regulates <strong>the</strong> chromosome replication cycle. Proc Natl Acad Sci U S A. 98, 11211-11217.<br />
Weinreich, M., Liang, C., Stillman, B. (1999) The Cdc6p nucleotide-binding motif is required for loading mcm<br />
proteins onto chromatin. Proc Natl Acad Sci U S A. 96, 441-446.<br />
Weitzer, S., Lehane, C., Uhlmann, F. (2003) A model for ATP hydrolysis-dependent binding of cohesin to DNA.<br />
Curr Biol. 13, 1930-1940.<br />
Williams, R.S., Shohet, R.V., Stillman, B. (1997) A human protein related to yeast Cdc6p. Proc Natl Acad Sci U S<br />
A. 94, 142-147.<br />
Wood, J.S., Hartwell, L.H. (1982) A dependent pathway of gene functions leading to chromosome segregation in<br />
Saccharomyces cerevisiae. J <strong>Cell</strong> Biol. 94, 718-726.<br />
Wotton, D., Shore, D. (1997) A novel Rap1p-interacting factor, Rif2p, cooperates with Rif1p to regulate telomere<br />
length in Saccharomyces cerevisiae. Genes Dev. 11, 748-760.<br />
Wu, M., Gerhart, J.C. (1980). Partial purification <strong>and</strong> characterization of <strong>the</strong> maturation-promoting factor from<br />
eggs of Xenopus laevis. Dev. Biol. 1980 79: 465–477.<br />
Wuarin, J., Nurse, P. (1996) Regulating S phase: CDKs, Licensing <strong>and</strong> proteolysis. <strong>Cell</strong> 85: 785–787.<br />
Wyrick, J.J., Aparicio, J.Gg., Chen, T., Barnett, J.D., Jennings, E.G., Young, R.A., Bell, S.P., Aparicio, O.M.<br />
Genomewide distribution of ORC <strong>and</strong> MCM proteins in S. cerevisiae: highresolution mapping of replication<br />
origins. Science 294 (2001) 2357-2360.<br />
Yamamoto, A., Guacci, V., Koshl<strong>and</strong>, D. (1996a) Pds1p is required for faithful execution of anaphase in <strong>the</strong> yeast,<br />
Saccharomyces cerevisiae. J <strong>Cell</strong> Biol 133, 85-97.<br />
Yamamoto, A., Guacci, V., Koshl<strong>and</strong>, D. (1996b) Pds1p, an inhibitor of anaphase in budding yeast, plays a critical<br />
role in <strong>the</strong> APC <strong>and</strong> checkpoint pathway(s). J <strong>Cell</strong> Biol 133, 99-1<strong>10</strong>.<br />
Yu, H.G., Koshl<strong>and</strong>, D. (2005) Chromosome morphogenesis: condensin-dependent cohesin removal during<br />
meiosis. <strong>Cell</strong> 123, 397-407.<br />
Zachariae, W., Nasmyth, K. (1999) Whose end is destruction: cell division <strong>and</strong> <strong>the</strong> anaphase-promoting complex.<br />
Genes Dev. 13, 2039-2058.<br />
Zachariae, W., Schwab, M., Nasmyth, K., Seufert, W. (1998) Control of cyclin ubiquitination by CDK-regulated<br />
binding of Hct1 to <strong>the</strong> anaphase promoting complex. Science 282, 1721-1724.<br />
Zachariae, W., Shin, T.H., Galova, M., Obermaier, B., Nasmyth, K. (1996) Identification of subunits of <strong>the</strong><br />
anaphase-promoting complex of Saccharomyces cerevisiae. Science 274, 1201-1204.<br />
Zhou, J., Monson, E.K., Teng, S.C., Schulz, V.P., Zakian, V.A. (2000) Pif1p helicase, a catalytic inhibitor of<br />
telomerase in yeast. Science. 289, 771-774.<br />
Zhu, H., Klemic, J.F., Chang, S., Bertone, P., Casamayor, A., Klemic, K.G., Smith, D., Gerstein, M., Reed, M.A.,<br />
Snyder, M. Analysis of yeast protein kinases using protein chips. Nat. Genet. 26 (2000) 283-289.<br />
Zou, L., Mitchell, J., Stillman, B. (1997) CDC45, a novel yeast gene that functions with <strong>the</strong> origin recognition<br />
complex <strong>and</strong> Mcm proteins in initiation of DNA replication. Mol <strong>Cell</strong> Biol. 17, 553-563.