Characterisation of phenolic extracts from olive pulp and ... - ESAC
Characterisation of phenolic extracts from olive pulp and ... - ESAC
Characterisation of phenolic extracts from olive pulp and ... - ESAC
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
SM Cardoso et al<br />
HO<br />
HO<br />
m/z 341<br />
OH<br />
O<br />
OH<br />
HO<br />
HO<br />
O<br />
HO<br />
HO<br />
Z<br />
O<br />
Z<br />
OH<br />
HO<br />
HO<br />
OH<br />
O<br />
OH<br />
HO<br />
HO<br />
Y<br />
O<br />
O<br />
OH<br />
O<br />
O<br />
(-H) -<br />
m/z 551 O<br />
Y<br />
(-H) - OH<br />
OH<br />
O<br />
(-H) -<br />
HO<br />
O<br />
OH<br />
- CO 2 HO<br />
OH<br />
O<br />
O OH<br />
OH<br />
m/z 389<br />
O<br />
O<br />
O<br />
OH<br />
O<br />
(-H) -<br />
HO<br />
O<br />
HO<br />
OH<br />
O OH<br />
m/z 507<br />
Z<br />
O<br />
OH<br />
O<br />
OH<br />
Y<br />
HO<br />
HO<br />
OH<br />
O<br />
O<br />
OH<br />
O<br />
m/z 345<br />
OH<br />
O<br />
(-H) -<br />
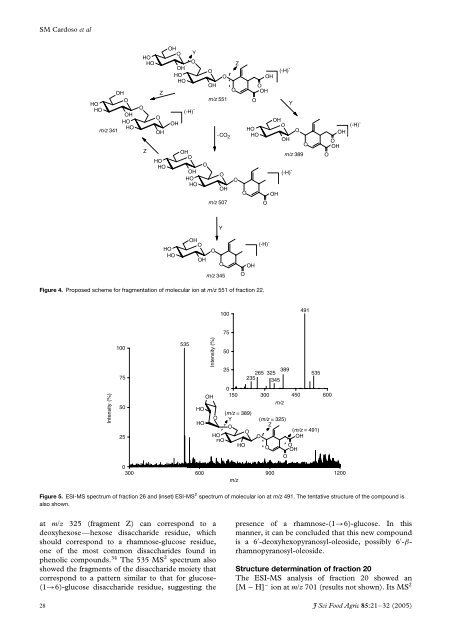
Figure 4. Proposed scheme for fragmentation <strong>of</strong> molecular ion at m/z 551 <strong>of</strong> fraction 22.<br />
100<br />
491<br />
75<br />
Intensity (%)<br />
100<br />
75<br />
50<br />
25<br />
535<br />
HO<br />
HO<br />
Intensity (%)<br />
OH<br />
O<br />
HO<br />
HO<br />
50<br />
25<br />
389<br />
265 325<br />
235 345<br />
535<br />
0<br />
150 300 450 600<br />
m/z<br />
(m/z = 389)<br />
Y<br />
O<br />
O<br />
HO<br />
(m/z = 325)<br />
Z<br />
O<br />
O<br />
O<br />
OH<br />
O<br />
(m/z = 491)<br />
OH<br />
0<br />
300 600 900 1200<br />
m/z<br />
Figure 5. ESI-MS spectrum <strong>of</strong> fraction 26 <strong>and</strong> (inset) ESI-MS 2 spectrum <strong>of</strong> molecular ion at m/z 491. The tentative structure <strong>of</strong> the compound is<br />
also shown.<br />
at m/z 325 (fragment Z) can correspond to a<br />
deoxyhexose—hexose disaccharide residue, which<br />
should correspond to a rhamnose-glucose residue,<br />
one <strong>of</strong> the most common disaccharides found in<br />
<strong>phenolic</strong> compounds. 34 The 535 MS 2 spectrum also<br />
showed the fragments <strong>of</strong> the disaccharide moiety that<br />
correspond to a pattern similar to that for glucose-<br />
(1→6)-glucose disaccharide residue, suggesting the<br />
presence <strong>of</strong> a rhamnose-(1→6)-glucose. In this<br />
manner, it can be concluded that this new compound<br />
is a 6 ′ -deoxyhexopyranosyl-oleoside, possibly 6 ′ -βrhamnopyranosyl-oleoside.<br />
Structure determination <strong>of</strong> fraction 20<br />
The ESI-MS analysis <strong>of</strong> fraction 20 showed an<br />
[M − H] − ion at m/z 701 (results not shown). Its MS 2<br />
28 J Sci Food Agric 85:21–32 (2005)