Primer on Pericyclic Reactions
Primer on Pericyclic Reactions
Primer on Pericyclic Reactions
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
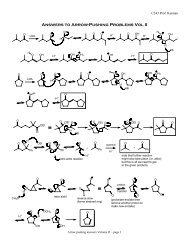
C343 Professor Kennan<br />
• Since adding <strong>on</strong>e π b<strong>on</strong>d (and thus two electr<strong>on</strong>s) makes a molecule’s HOMO <strong>on</strong>e level higher, and<br />
since the symmetries alternate, the HOMO of hexatriene (below) must be symmetric. Thus the reacti<strong>on</strong><br />
is disrotatory. If you add two more π b<strong>on</strong>ds (to get 5) then the HOMO will flip from symmetric to<br />
antisymmetric (for 4 π b<strong>on</strong>ds) and back to symmetric. Since this alternati<strong>on</strong> c<strong>on</strong>tinues indefinitely, all<br />
c<strong>on</strong>jugated π systems with an odd number of double b<strong>on</strong>ds will have symmetric HOMOs. Put more<br />
succinctly:<br />
Molecules with an even number of π b<strong>on</strong>ds (4n electr<strong>on</strong>s) in the ring opened form have antisymmetric<br />
HOMOs, while those with an odd number of π b<strong>on</strong>ds (4n+2 electr<strong>on</strong>s) have symmetric HOMOs<br />
6π electr<strong>on</strong>s => Ψ 3 is now the HOMO<br />
orbitals alternate symmetry => if Ψ 2 is antisymmetric Ψ 3 must be symmetric<br />
symmetric => disrotatory<br />
• Similarly, since photochemical c<strong>on</strong>diti<strong>on</strong>s involve promoti<strong>on</strong> of <strong>on</strong>e electr<strong>on</strong> the effect is the same as<br />
adding a new π b<strong>on</strong>d – in other words the next highest orbital now becomes the HOMO. Since the<br />
symmetry alternates, this has the effect of switching between c<strong>on</strong>rotatory and disrotatory. Thus:<br />
Reacti<strong>on</strong>s which are c<strong>on</strong>rotatory thermally will be disrotatory photochemically and vise versa<br />
new LUMO<br />
opposite<br />
symmetries<br />
by definiti<strong>on</strong><br />
LUMO<br />
HOMO<br />
hν new HOMO => change in symmetry<br />
• So now we can determine if any reacti<strong>on</strong> is c<strong>on</strong>rotatory or disrotatory. Start with the number of π b<strong>on</strong>ds,<br />
and determine if it is like butadiene (even number π b<strong>on</strong>ds, 4n electr<strong>on</strong>s) or not. Remember or figure<br />
out that butadiene is antisymmetric/c<strong>on</strong>rotatory (thermally). Then ask two questi<strong>on</strong>s:<br />
(1) Is it like butadiene? antisymmetric/c<strong>on</strong>rotatory; if not symmetric/disrotatory.<br />
(2) Is it thermal (heat)? leave above decisi<strong>on</strong> al<strong>on</strong>e; if photochemical (light) switch to opposite<br />
II. Cycloadditi<strong>on</strong>s<br />
• Count the π b<strong>on</strong>ds/electr<strong>on</strong>s in each molecule<br />
• Determine if the HOMOs for each molecule are symmetric or antisymmetric as above<br />
• The LUMO for each molecule will have the opposite symmetry to the HOMO (since it alternates)<br />
• Compare HOMO of molecule A with LUMO of molecule B (doesn’t matter how you assign A/B)<br />
If the HOMO of A and the LUMO of B have the same symmetry, the reacti<strong>on</strong> is “allowed”<br />
If the HOMO of A and the LUMO of B have the opposite symmetry, the reacti<strong>on</strong> is “forbidden”<br />
• Since photochemical (light) c<strong>on</strong>diti<strong>on</strong>s involve altering the symmetry of molecule A or B (not both):<br />
Thermally allowed (heat) cycloadditi<strong>on</strong>s are photochemically (light) forbidden and vise versa