Frustrated Lewis Pairs-2 - Franklin Chm Colostate
Frustrated Lewis Pairs-2 - Franklin Chm Colostate
Frustrated Lewis Pairs-2 - Franklin Chm Colostate
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
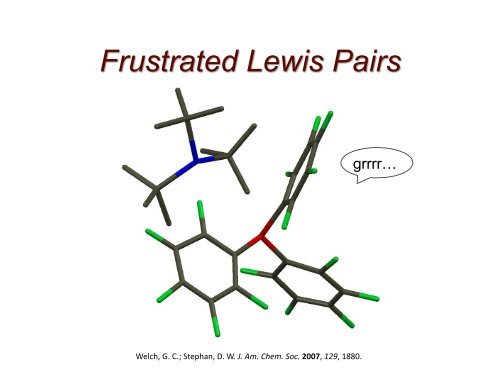
Welch, G. C.; Stephan, D. W. J. Am. Chem. Soc. 2007, 129, 1880.<br />
grrrr…
• Background<br />
Outline<br />
• The Beginning: H 2 AcCvaCon<br />
• Metal-‐Free HydrogenaCon<br />
• AddiCons to Unsaturated Compounds<br />
• CO 2 AcCvaCon
<strong>Lewis</strong> Acids and Bases<br />
“... we may say that a basic substance is one which has a<br />
lone pair of electrons which may be used to complete<br />
the stable group of another atom, and that an acid<br />
substance is one which can employ a lone pair from<br />
another molecule in comple@ng the stable group of one<br />
of its own atoms.”<br />
Gilbert N. <strong>Lewis</strong><br />
base = e -‐ pair donor<br />
acid = e -‐ pair acceptor<br />
G. N. <strong>Lewis</strong>, Valence and the Structure of Atoms and Molecules, Chemical Catalogue Company, New York, 1923.
<strong>Lewis</strong> Acids and Bases<br />
base = e -‐ pair donor<br />
acid = e -‐ pair acceptor
Brown:<br />
WiMg:<br />
Tochtermann:<br />
FLPs in History<br />
Brown, H. C.; Schlesinger, H. I.; Cardon, S. Z. J. Am. Chem. Soc. 1942, 64, 325.<br />
WiWg, G.; Benz, E. Chem. Ber. 1959, 92, 1999.<br />
Tochtermann, W. Angew. Chem. Int. Ed. Engl. 1966, 5, 351.
New ReacCvity<br />
first reversible H 2 ac@va@on by a non-‐transi@on metal system<br />
Welch, G. C.; San Juan, R. R.; Masuda, J. D.; Stephan, D. W. Science 2006, 314, 1124.
H 2 AcCvaCon by Main Group Compounds<br />
Spikes, G. H.; FeWnger, J. C.; Power, P. P. J. Am. Chem. Soc. 2005, 127, 12232.<br />
Frey, G. D.; Lavallo, V.; Donnadieu, B.; Schoeller, W.; Bertrand, G. Science 2007, 316, 439.<br />
Welch, G. C.; San Juan, R. R.; Masuda, J. D.; Stephan, D. W. Science 2006, 314, 1124.
H 2 Cleavage by Phosphines and Boranes<br />
strict steric and electronic requirements for H 2 ac@va@on<br />
Welch, G. C.; Stephan, D. W. J. Am. Chem. Soc. 2007, 129, 1880.
Mechanism of H 2 AcCvaCon<br />
termolecular collision = entropically disfavored<br />
H 2 adduct formaCon disfavored due to Pauli repulsion<br />
weak associaCon of LB and LA generates a ‘frustrated complex’<br />
Rokob, T. A.; Hamza, A.; SCrling, A.; Soós, T.; Pápai, I. Angew. Chem. Int. Ed. 2008, 47, 2435.
H 2 Cleavage by the <strong>Frustrated</strong> Complex<br />
• stabilizaCon energy of -‐11.5 kcal/mol<br />
• dispersion interacCons<br />
• mulCple C-‐H F hydrogen bonds<br />
• planar BC 3 unit = no charge transfer<br />
• structural flexibility allows H 2 access<br />
• H-‐H polarizaCon in TS<br />
• simultaneous tBu 3 P σ*(H 2 ) and σ<br />
(H 2 ) B(C 6 F 5 ) 3 leads to H-‐H cleavage<br />
Rokob, T. A.; Hamza, A.; SCrling, A.; Soós, T.; Pápai, I. Angew. Chem. Int. Ed. 2008, 47, 2435.
Bridged Phosphine-‐Borane Adduct<br />
Organometallics 2008, 27, 1135.<br />
reversible adduct forma@on allows for H 2 ac@va@on<br />
Stephan, D. W. Chem. Commun. 2007, 5072. Erker, G. Dalton Trans. 2009, 1534.
Reversible H 2 AcCvaCon<br />
Organometallics<br />
1999, 18, 1724<br />
2.08Å<br />
proton lability leads to reversible ac@va@on?<br />
Wang, H.; Fröhlich, R.; Kehr, G.; Erker, G. Chem. Comm. 2008, 5966.
Reversibility: H 2 EliminaCon
Reversible H 2 AcCvaCon by Design<br />
balance of acidity and basicity<br />
Ullrich, M.; Lough, A. J.; Stephan, D. W. J. Am. Chem. Soc. 2009, 131, 52.<br />
Dalton Trans. 2007, 3407.
H 2 AcCvaCon: FLPs as TransiCon Metals<br />
small energy separa@on between fron@er orbitals<br />
Power, P. P. Nature 2010, 463, 171; Kubas, G. J. Chem. Rev. 2007, 107, 4152; Pápai, I. Int. J. Quant. Chem. 2009, 109, 2416.
<strong>Frustrated</strong> <strong>Lewis</strong> <strong>Pairs</strong> Today<br />
R 3 X / ER’ 3<br />
PR 3 , NR 3 , BR’ 3 , AlR’ 3<br />
NHR 2 , :CR 2
• Background<br />
Outline<br />
• The Beginning: H 2 AcCvaCon<br />
• Metal-‐Free HydrogenaCon<br />
• AddiCons to Unsaturated Compounds<br />
• CO 2 AcCvaCon
First Metal-‐free CatalyCc HydrogenaCon<br />
Chase, P. A.; Welch, G. C.; Jurca, T.; Stephan, D. W. Angew. Chem. Int. Ed. 2007, 46, 8050.
Imine HydrogenaCon<br />
Sumerin, V.; Schulz, F.; Atsumi, M.; Wang, C.; Nieger, M.; Leskelä, M.; Repo, T.; Pyykkö, P.; Rieger, B. J. Am. Chem. Soc. 2008, 130, 14117.
Silyl Enol Ether HydrogenaCon<br />
Wang, H.; Frölich, R.; Kehr, G.; Erker, G. Chem. Comm. 2008, 5966.
HydrogenaCon by the Bridged FLP<br />
Spies, P.; Schwendemann, S.; Lange, S.; Kehr, G.; Frölich, R.; Erker, G. Angew. Chem. Int. Ed. 2008, 47,7543.
Organometallic Substrates<br />
Schwendemann, S.; Asli Tumay, T.; Axenov, K. V.; Peuser, I.; Kehr, G.; Frölich, R.; Erker, G. Organometallics 2010, 29, 1067.
<strong>Frustrated</strong> Substrates<br />
Chase, P. A.; Jurca, T.; Stephan, D. W. Chem. Commun. 2008, 1701. Chen, D.; Klankermayer, J. Chem. Commun. 2008, 2130.
• Background<br />
Outline<br />
• The Beginning: H 2 AcCvaCon<br />
• Metal-‐Free HydrogenaCon<br />
• AddiCons to Unsaturated Compounds<br />
• CO 2 AcCvaCon
1,2 AddiCon to Carbonyl Groups<br />
Mömming, C. M.; Frömel, S.; Kehr, G.; Fröhlich, R.; Grimme, S.; Erker, G. J. Am. Chem. Soc. 2009, 131, 12280.
AddiCons to Olefins<br />
McCahill, J. S. J.; Welch, G. C.; Stephan, D. W. Angew. Chem. Int. Ed. 2007, 46, 4968.<br />
Voss, T.; Chen, C.; Kehr, G.; Nauha, E.; Erker, G.; Stephan, D. W. Chem. Eur. J. 2010, 16, 3005.
1,4 AddiCon to Conjugated Systems<br />
Ullrich, M.; Seto, K. S. H., Lough, A. J.; Stephan, D. W. Chem. Comm. 2009, 2335.<br />
Mömming, C.; Kehr, G.; Wibbeling, B.; Fröhlich, R.; Schirmer, B.; Grimme, S.; Erker, G. Angew. Chem. Int. Ed. 2010, 49, 2414.
Terminal Alkyne AcCvaCon<br />
Dureen, M. A.; Stephan, D. W. J. Am. Chem. Soc. 2009, 131, 8396. Jiang et al. Organometallics 2010, 29, 125.
• Background<br />
Outline<br />
• The Beginning: H 2 AcCvaCon<br />
• Metal-‐Free HydrogenaCon<br />
• AddiCons to Unsaturated Compounds<br />
• CO 2 AcCvaCon
CO 2 AcCvaCon and ReducCon<br />
Mömming, C. M.; Oten, E.; Kehr, G.; Fröhlich, R.; Grimme, S.; Stephan, D. W.; Erker, G. Angew. Chem. Int. Ed. 2009, 48, 6643.<br />
Ashley, A. E.; Thompson, A. L.; O’Hare, D. Angew. Chem. Int. Ed. 2009, 48, 9839.
Conclusion<br />
• CombinaCon of unquenched acidity and<br />
basicity lends unique reacCvity to frustrated<br />
<strong>Lewis</strong> pairs<br />
• FLP small molecule acCvaCon and reacCons<br />
with π systems suggest a parallel with<br />
transiCon metal chemistry<br />
• FLP catalysis provides a mild, metal-‐free<br />
alternaCve for hydrogenaCon