KEY-FinalExamStudyGuide_Fall2013
KEY-FinalExamStudyGuide_Fall2013
KEY-FinalExamStudyGuide_Fall2013
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
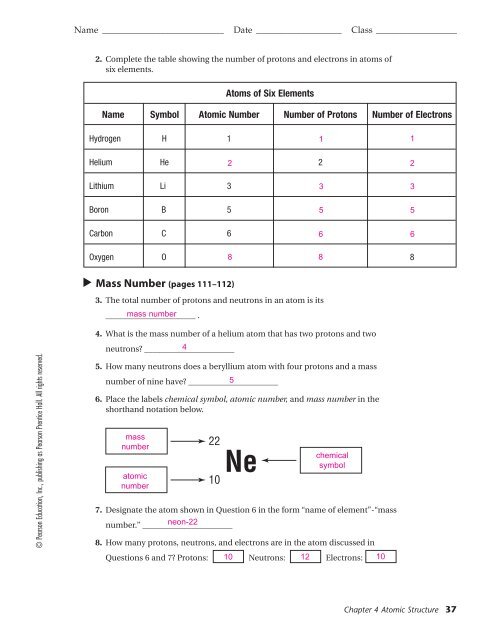
Name ___________________________ Date ___________________ Class __________________<br />
2. Complete the table showing the number of protons and electrons in atoms of<br />
six elements.<br />
Atoms of Six Elements<br />
Name Symbol Atomic Number Number of Protons Number of Electrons<br />
Hydrogen H 1<br />
1 1<br />
Helium He 2<br />
2<br />
2<br />
Lithium Li 3<br />
Boron B 5<br />
Carbon C 6<br />
3<br />
5<br />
6<br />
3<br />
5<br />
6<br />
Oxygen O 8 8<br />
8<br />
Mass Number (pages 111–112)<br />
3. The total number of protons and neutrons in an atom is its<br />
______________________ mass number .<br />
© Pearson Education, Inc., publishing as Pearson Prentice Hall. All rights reserved.<br />
4. What is the mass number of a helium atom that has two protons and two<br />
neutrons? ______________________<br />
4<br />
5. How many neutrons does a beryllium atom with four protons and a mass<br />
number of nine have? ______________________<br />
5<br />
6. Place the labels chemical symbol, atomic number, and mass number in the<br />
shorthand notation below.<br />
mass<br />
number<br />
atomic<br />
number<br />
22<br />
Ne<br />
10<br />
chemical<br />
symbol<br />
7. Designate the atom shown in Question 6 in the form “name of element”-“mass<br />
number.” ______________________<br />
neon-22<br />
8. How many protons, neutrons, and electrons are in the atom discussed in<br />
Questions 6 and 7? Protons: 10 Neutrons: 12 Electrons: 10<br />
Chapter 4 Atomic Structure 37