KEY-FinalExamStudyGuide_Fall2013
KEY-FinalExamStudyGuide_Fall2013
KEY-FinalExamStudyGuide_Fall2013
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
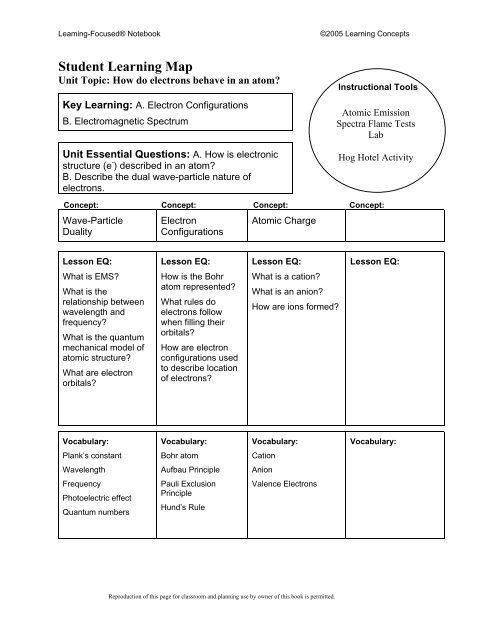
Leaming-Focused® Notebook<br />
©2005 Learning Concepts<br />
Student Learning Map<br />
Unit Topic: How do electrons behave in an atom?<br />
Key Learning: A. Electron Configurations<br />
B. Electromagnetic Spectrum<br />
Unit Essential Questions: A. How is electronic<br />
structure (e - ) described in an atom?<br />
B. Describe the dual wave-particle nature of<br />
electrons.<br />
Instructional Tools<br />
Atomic Emission<br />
Spectra Flame Tests<br />
Lab<br />
Hog Hotel Activity<br />
Concept: Concept: Concept: Concept:<br />
Wave-Particle<br />
Duality<br />
Electron<br />
Configurations<br />
Atomic Charge<br />
Lesson EQ:<br />
Lesson EQ:<br />
Lesson EQ:<br />
Lesson EQ:<br />
What is EMS?<br />
What is the<br />
relationship between<br />
wavelength and<br />
frequency?<br />
What is the quantum<br />
mechanical model of<br />
atomic structure?<br />
What are electron<br />
orbitals?<br />
How is the Bohr<br />
atom represented?<br />
What rules do<br />
electrons follow<br />
when filling their<br />
orbitals?<br />
How are electron<br />
configurations used<br />
to describe location<br />
of electrons?<br />
What is a cation?<br />
What is an anion?<br />
How are ions formed?<br />
Vocabulary:<br />
Vocabulary:<br />
Vocabulary:<br />
Vocabulary:<br />
Plank’s constant<br />
Bohr atom<br />
Cation<br />
Wavelength<br />
Aufbau Principle<br />
Anion<br />
Frequency<br />
Photoelectric effect<br />
Quantum numbers<br />
Pauli Exclusion<br />
Principle<br />
Hund’s Rule<br />
Valence Electrons<br />
Reproduction of this page for classroom and planning use by owner of this book is permitted.