KEY-FinalExamStudyGuide_Fall2013
KEY-FinalExamStudyGuide_Fall2013
KEY-FinalExamStudyGuide_Fall2013
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
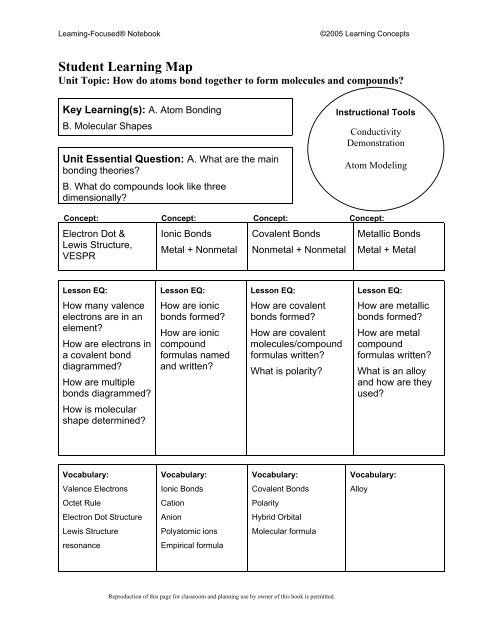
Leaming-Focused® Notebook<br />
©2005 Learning Concepts<br />
Student Learning Map<br />
Unit Topic: How do atoms bond together to form molecules and compounds?<br />
Key Learning(s): A. Atom Bonding<br />
B. Molecular Shapes<br />
Unit Essential Question: A. What are the main<br />
bonding theories?<br />
B. What do compounds look like three<br />
dimensionally?<br />
Instructional Tools<br />
Conductivity<br />
Demonstration<br />
Atom Modeling<br />
Concept: Concept: Concept: Concept:<br />
Electron Dot & Ionic Bonds Covalent Bonds Metallic Bonds<br />
Lewis Structure,<br />
VESPR<br />
Metal + Nonmetal Nonmetal + Nonmetal Metal + Metal<br />
Lesson EQ:<br />
Lesson EQ:<br />
Lesson EQ:<br />
Lesson EQ:<br />
How many valence<br />
electrons are in an<br />
element?<br />
How are electrons in<br />
a covalent bond<br />
diagrammed?<br />
How are multiple<br />
bonds diagrammed?<br />
How are ionic<br />
bonds formed?<br />
How are ionic<br />
compound<br />
formulas named<br />
and written?<br />
How are covalent<br />
bonds formed?<br />
How are covalent<br />
molecules/compound<br />
formulas written?<br />
What is polarity?<br />
How are metallic<br />
bonds formed?<br />
How are metal<br />
compound<br />
formulas written?<br />
What is an alloy<br />
and how are they<br />
used?<br />
How is molecular<br />
shape determined?<br />
Vocabulary:<br />
Vocabulary:<br />
Vocabulary:<br />
Vocabulary:<br />
Valence Electrons<br />
Ionic Bonds<br />
Covalent Bonds<br />
Alloy<br />
Octet Rule<br />
Cation<br />
Polarity<br />
Electron Dot Structure<br />
Anion<br />
Hybrid Orbital<br />
Lewis Structure<br />
Polyatomic ions<br />
Molecular formula<br />
resonance<br />
Empirical formula<br />
Reproduction of this page for classroom and planning use by owner of this book is permitted.