Monitoring of Suspected Adverse Drug Reactions in Oncology Unit ...
Monitoring of Suspected Adverse Drug Reactions in Oncology Unit ...
Monitoring of Suspected Adverse Drug Reactions in Oncology Unit ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
International Journal <strong>of</strong> Research <strong>in</strong> Pharmaceutical and Biomedical Sciences ISSN: 2229-3701<br />
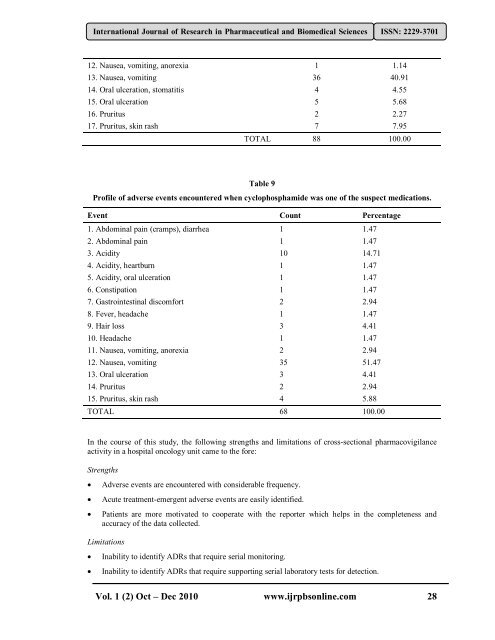
12. Nausea, vomit<strong>in</strong>g, anorexia 1 1.14<br />
13. Nausea, vomit<strong>in</strong>g 36 40.91<br />
14. Oral ulceration, stomatitis 4 4.55<br />
15. Oral ulceration 5 5.68<br />
16. Pruritus 2 2.27<br />
17. Pruritus, sk<strong>in</strong> rash 7 7.95<br />
TOTAL 88 100.00<br />
Table 9<br />
Pr<strong>of</strong>ile <strong>of</strong> adverse events encountered when cyclophosphamide was one <strong>of</strong> the suspect medications.<br />
Event Count Percentage<br />
1. Abdom<strong>in</strong>al pa<strong>in</strong> (cramps), diarrhea 1 1.47<br />
2. Abdom<strong>in</strong>al pa<strong>in</strong> 1 1.47<br />
3. Acidity 10 14.71<br />
4. Acidity, heartburn 1 1.47<br />
5. Acidity, oral ulceration 1 1.47<br />
6. Constipation 1 1.47<br />
7. Gastro<strong>in</strong>test<strong>in</strong>al discomfort 2 2.94<br />
8. Fever, headache 1 1.47<br />
9. Hair loss 3 4.41<br />
10. Headache 1 1.47<br />
11. Nausea, vomit<strong>in</strong>g, anorexia 2 2.94<br />
12. Nausea, vomit<strong>in</strong>g 35 51.47<br />
13. Oral ulceration 3 4.41<br />
14. Pruritus 2 2.94<br />
15. Pruritus, sk<strong>in</strong> rash 4 5.88<br />
TOTAL 68 100.00<br />
In the course <strong>of</strong> this study, the follow<strong>in</strong>g strengths and limitations <strong>of</strong> cross-sectional pharmacovigilance<br />
activity <strong>in</strong> a hospital oncology unit came to the fore:<br />
Strengths<br />
<br />
<br />
<br />
<strong>Adverse</strong> events are encountered with considerable frequency.<br />
Acute treatment-emergent adverse events are easily identified.<br />
Patients are more motivated to cooperate with the reporter which helps <strong>in</strong> the completeness and<br />
accuracy <strong>of</strong> the data collected.<br />
Limitations<br />
<br />
<br />
Inability to identify ADRs that require serial monitor<strong>in</strong>g.<br />
Inability to identify ADRs that require support<strong>in</strong>g serial laboratory tests for detection.<br />
Vol. 1 (2) Oct – Dec 2010 www.ijrpbsonl<strong>in</strong>e.com 28