medical device or a medicinal product
medical device or a medicinal product
medical device or a medicinal product
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
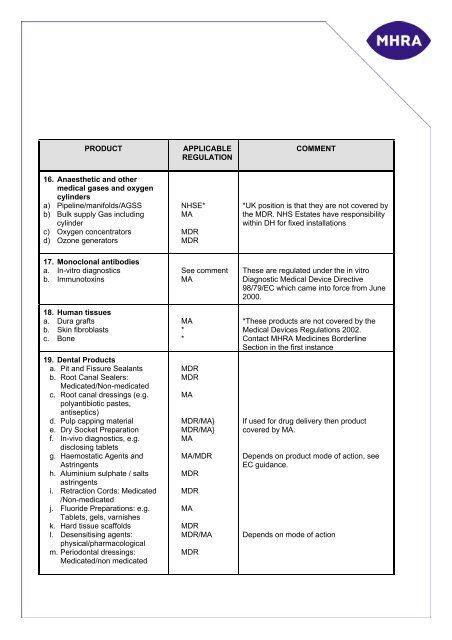
PRODUCT<br />
APPLICABLE<br />
REGULATION<br />
COMMENT<br />
16. Anaesthetic and other<br />
<strong>medical</strong> gases and oxygen<br />
cylinders<br />
a) Pipeline/manifolds/AGSS<br />
b) Bulk supply Gas including<br />
cylinder<br />
c) Oxygen concentrat<strong>or</strong>s<br />
d) Ozone generat<strong>or</strong>s<br />
NHSE*<br />
MA<br />
MDR<br />
MDR<br />
*UK position is that they are not covered by<br />
the MDR. NHS Estates have responsibility<br />
within DH f<strong>or</strong> fixed installations<br />
17. Monoclonal antibodies<br />
a. In-vitro diagnostics<br />
b. Immunotoxins<br />
18. Human tissues<br />
a. Dura grafts<br />
b. Skin fibroblasts<br />
c. Bone<br />
19. Dental Products<br />
a. Pit and Fissure Sealants<br />
b. Root Canal Sealers:<br />
Medicated/Non-medicated<br />
c. Root canal dressings (e.g.<br />
polyantibiotic pastes,<br />
antiseptics)<br />
d. Pulp capping material<br />
e. Dry Socket Preparation<br />
f. In-vivo diagnostics, e.g.<br />
disclosing tablets<br />
g. Haemostatic Agents and<br />
Astringents<br />
h. Aluminium sulphate / salts<br />
astringents<br />
i. Retraction C<strong>or</strong>ds: Medicated<br />
/Non-medicated<br />
j. Flu<strong>or</strong>ide Preparations: e.g.<br />
Tablets, gels, varnishes<br />
k. Hard tissue scaffolds<br />
l. Desensitising agents:<br />
physical/pharmacological<br />
m. Periodontal dressings:<br />
Medicated/non medicated<br />
See comment<br />
MA<br />
MA<br />
*<br />
*<br />
MDR<br />
MDR<br />
MA<br />
MDR/MA}<br />
MDR/MA}<br />
MA<br />
MA/MDR<br />
MDR<br />
MDR<br />
MA<br />
MDR<br />
MDR/MA<br />
MDR<br />
These are regulated under the in vitro<br />
Diagnostic Medical Device Directive<br />
98/79/EC which came into f<strong>or</strong>ce from June<br />
2000.<br />
*These <strong>product</strong>s are not covered by the<br />
Medical Devices Regulations 2002.<br />
Contact MHRA Medicines B<strong>or</strong>derline<br />
Section in the first instance<br />
If used f<strong>or</strong> drug delivery then <strong>product</strong><br />
covered by MA.<br />
Depends on <strong>product</strong> mode of action, see<br />
EC guidance.<br />
Depends on mode of action